Translate this page into:
Link between ACE I/D Gene Polymorphism and Dyslipidemia in Diabetic Nephropathy: A Case-control Study from Hyderabad, India
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Diabetic nephropathy (DN) is the commonest single cause of end-stage renal failure, and dyslipidemia is a critical risk factor in the occurrence of DN. In the light of recent reports emphasizing the importance of angiotensin I-converting enzyme (ACE) in the modulation of plasma lipids, we sought to evaluate the influence of ACE I/D gene polymorphism with dyslipidemia status among type 2 diabetic (T2D) patients with and without nephropathy in the genetic predisposition and the progression to DN.
Method:
This study comprised of 600 subjects, which include patients with DN, T2D, and healthy controls (HC). Polymerase chain reaction based genotyping of ACE I/D polymorphism was performed and appropriate statistical analysis was done.
Results:
Out of the 600 subjects, 20 (10%) of the HC, 73 (36.5%) of the T2D group, and 125 (62.5%) of the DN subjects had dyslipidemia. The D allele (0.62) and DD (42.5) genotype frequencies were higher in the DN group in comparison with T2D and HC (P < 0.05). The genotypes also varied among patients with dyslipidemia (χ2 5.04; P < 0.05) but not in the non-dyslipidemia group. Under the co-dominant model, DD genotype conferred a risk of 1.26 (P < 0.001) toward DN, whereas the ID genotype offered protection from DN among the dyslipidemic subjects (OR = 0.05; P < 0.01). In addition, genotype-dependent difference was seen in the plasma lipid levels among study groups. A multiple logistic regression analysis revealed male gender, BMI, HbA1c, TG, HDL, and ACE DD genotype as independent risk factors for the development of DN.
Conclusion:
The study showed a significant predisposing association of ACE DD genotype with DN and protective effect of ID genotype on DN in the dyslipidemia subgroup.
Keywords
ACE I/D polymorphism
diabetic nephropathy
dyslipidemia
type 2 diabetes
Introduction
Diabetes mellitus (DM) is a multifactorial metabolic disorder caused as a consequence of defects in insulin secretion and insulin action. Of the major forms of DM, type 2 diabetes mellitus (T2D) is the most common type, constituting nearly 85%–95% of the DM cases. Longstanding diabetes results in a multitude of complications of which diabetic nephropathy (DN) is an important microvascular complication observed in almost 40% of DM individuals.[1234] Numerous genetic variants and environmental factors are proposed to be involved in the etiology of DN. However, the precise mechanism regarding the pathophysiology of DN remains poorly understood.[56]
Existing literature suggests that the renin-angiotensin system (RAS), which plays an important role in the regulation of blood pressure and fluid electrolyte homeostasis, is an active mediator of progressive renal injury in DN. Moreover, insulin resistance and hyperinsulinemia together with components of the RAS have been demonstrated to lead to DN. It is well recognized that alteration in the lipid metabolism results from insulin resistance and defective insulin action,[78] and resultant dyslipidemia has been shown to be associated with increased urinary albumin excretion rate, a predictive marker for progressive renal failure.[9] Previous animal studies have demonstrated that albuminuria is exaggerated by hypercholesterolemia. Diabetic dyslipidemia has been shown to increase excessive extracellular matrix (ECM) production and macrophage infiltration in the glomeruli under hyperglycemic condition.[910] In addition, lipid abnormalities in DN were shown to get more emphasized with deteriorating renal function and urinary albumin excretion.[1112]
Angiotensin I-converting enzyme (ACE), a crucial component of RAS, is synthesized in epithelial and endothelial cells located in kidneys, lungs, and blood vessels. It catalyzes the production of vasoactive peptide angiotensin II from its precursor angiotensin I and inactivates bradykinin. Angiotensin II plays a critical role in the maintenance of blood pressure, sodium homeostasis, and renal hemodynamics.[13] Aside from this role, emerging evidence proposes that the RAS and its components including ACE are implicated in the pathogenesis of T2D, by promoting the development of dyslipidemia, insulin resistance, and interfering with insulin signaling. The genes involved in the RAS are thus considered as important predisposing factors in the development of DN.
The ACE gene located on chromosome 17q23, spans approximately 21 kb, includes 26 exons and 25 introns and encodes a 1,306 amino acid protein. A well-characterized 287bp Alu repeat Insertion/Deletion polymorphism (rs1799752) has been identified in this gene, which has been studied extensively with renal and cardiovascular complications of T2D.[1415] Former studies report that the D allele is characterized by elevated plasma concentrations of ACE compared with that of I allele.[1617] Lee and Tsai revealed that individuals with DD genotype have a significantly higher prevalence of dyslipidemia and higher serum triglyceride levels than those with II genotypes.[18] However, to date, reports on the association between ACE I/D genotypes, dyslipidemia, and DN are sparse. Extensive literature survey turned up one article wherein combined effects of atherosclerotic risk factors such as hypertension, smoking, dyslipidemia, and ACE genotypes were analyzed for their impact on microalbuminuria.[9] With this premise, we proposed to categorize our DN subjects according to dyslipidemic status and explore the independent influence of the ACE I/D polymorphism on progression to DN.
This case-control study has thus been designed to not only investigate the association of ACE I/D gene polymorphism in the development of T2D and DN but also to analyze the influence of this polymorphic variant on DN in the presence and absence of dyslipidemia.
Materials and Methodology
Subjects
The study was conducted in a cohort of 600 subjects comprising of 200 healthy individuals, 200 T2D individuals without nephropathy, and 200 DN patients visiting Nizam's Institute of Medical Sciences and Princess Esra Hospital Hyderabad, India. The study was approved by the Ethics committee of Osmania University, Hyderabad, India. Demographic and anthropometric details such as age, gender, height, weight, family history of disease, and duration of diabetes were recorded in a pre-designed proforma. Written informed consent prior to the sample collection from all the participating individuals was obtained. The inclusion criteria for the three different study groups were (a) DN patients: Individuals diagnosed with a urine albumin excretion of >300 mg/day on two different occasions in a span of 3–6 months without any clinical evidence of other kidney diseases, infectious condition; (b) T2D individuals without nephropathy: Urinary albumin excretion of <30 mg/day (checked on three different occasions) and, longstanding diabetes for a period of 10 years with no history of hypertension prior to the development of diabetes; and (c) HC: Unrelated healthy individuals with no history of diabetes, hypertension, and renal disorders. Height and body weight were measured and body mass index (BMI) was calculated as body weight in kilogram divided by the square of body height in meter. A value ≥23 kg/mg2 was considered as a cut-off for being obese. Waist and hip circumference were measured; waist to hip ratio (WHR) was then calculated. A cut-off value of ≥0.88 in males and ≥0.81 in females was considered for classification of study subjects according to abdominal obesity.[19] Dyslipidemia was defined as elevated levels of triglycerides (TG), low-density lipoprotein, and reduced levels of high-density lipoprotein cholesterol.[20]
Biochemical analysis
All the clinical parameters were performed using standard techniques (Rapid diagnostics Olso Norway and Span diagnostics). The levels of fasting and post-lunch blood sugar were acquired from the medical records of the hospital.
Molecular analysis
Genomic DNA was extracted from the peripheral blood leukocytes, using DNA extraction kit (Hi-media, India) as per instructions of the manufacturer and stored at –20°C until further use. The ACE genotyping was performed by polymerase chain reaction (PCR) following Wali et al., 2012 protocol.[21] PCR products were resolved on 2% agarose gel, PCR of 490 bp indicates a genotype homozygous for insertion (II), 190 bp homozygous for deletion (DD), and presence of both 490 and 190 bp indicates heterozygosity ID [Figure 1].

- Agarose gel image illustrating homozygous DD, homozygous II and heterozygous ID genotype
Statistical analysis
The statistical test was performed using MedCalc software 14.8.1.0. The Student's t test was used to test for differences in various characteristics for continuous variables. The evaluation of data among the groups was performed by ANOVA (one-way and two-way ANOVA) test. Genotypic and allelic frequencies among study participants were analyzed with the Chi-square test, and Hardy-Weinberg equilibrium (HWE) was calculated. The association between genotypes was assessed by calculating the odds ratio (OR) at 95% confidence interval (CI). In addition, the risk conferred by ACE I/D polymorphism to DN under various genetic models was performed using SNP Stats online tool. A P value of <0.05 was considered statistically significant. Relationship between the risks factors and DN was assessed through multiple logistic regression (MLR).
Results
A total of 600 subjects were recruited for the present study. The baseline anthropometric and clinical features of the study population are summarized in Table 1. Results were expressed as mean ± SD in HC, T2D, and DN. A perusal of the table reveals that the greatest proportion of subjects with dyslipidemia was present in DN group followed by the T2D group and the HC group had the least proportion of subjects with dyslipidemia.
| HC (n=200) X±SD | T2D (n=200) X±SD | DN (n=200) X±SD | P | |
|---|---|---|---|---|
| Age (years) | 49.53±7.03 | 58.47±9.62 | 52.86±7.39 | <0.001 |
| Males (%) | 89 (44.5) | 96 (48) | 139 (69.5) | |
| Females (%) | 111 (55.5) | 104 (52) | 61 (30.5) | |
| Family history of diabetes | - | 95 (45.7) | 101 (50.5) | |
| Family history of nephropathy | - | 30 (15) | ||
| Mean duration of diabetes (years) | - | 14.03±5.14 | 12.69±7.05 | <0.05a |
| BMI (Kg/m2) | 23.23±3.27 | 24.08±2.10 | 25.01±3.29 | <0.001 |
| W/H ratio | 0.80±0.07 | 0.93±0.06 | 0.89±0.03 | <0.001 |
| HbA1c (%) | 4.81±0.45 | 7.56±0.85 | 8.20±1.21 | <0.001 |
| Serum creatinine (mg/dl) | 0.81±0.21 | 1.22±0.70 | 4.71±2.90 | <0.001 |
| Serum albumin (g/dl) | 4.20±0.51 | 4.41±0.59 | 3.01±0.71 | <0.001 |
| Blood urea (mg/dl) | 22.58±15.73 | 33.92±9.36 | 78.92±37.79 | <0.001 |
| Total cholesterol (mg/dl) | 152.05±27.42 | 183.60±48.33 | 197.20±49.98 | <0.001 |
| High-density lipoprotein (mg/dl) | 49.33±5.32 | 39.91±10.87 | 33.25±11.69 | <0.001 |
| Low-density lipoprotein (md/dl) | 65.03±63.75 | 114.47±47.69 | 131.28±53.93 | <0.001 |
| Very low-density lipoprotein | 22.17±5.07110.87±46.92 | 25.17±10.05 | 30.06±9.87 | <0.001 |
| Triglycerides (mg/dl) | 3.10±0.52 | 156.89±59.25 | 185.63±61.03 | <0.001 |
| TC/HDL ratio | 20 (10) | 5.11±2.43 | 6.85±3.38 | <0.001 |
| Dyslipidemia (%) | - | 73 (36.5) | 125 (62.5) | |
| Proteinuria (mg/dl) | - | 699.20±57.19b |
One-way ANOVA analysis for all the variables (except mean duration of diabetes) was performed as a test of significance. . a: t test P value, b: mean±SE
Table 2 represents the distribution of ACE I/D genotypes and their allelic frequencies among the studied population. The current analysis revealed that the percentage distribution of DD genotypes was more in DN (42.5%) when compared with T2D (25%) and HC (18%) subjects. The frequency of ID genotypes was marginally high among T2D (48.5%) than DN (40.5%) and HC (37.5%). Higher frequency of II genotype was found among HC (44.5%) than T2D (26.5%) and DN (18.7%) individuals. The ACE I/D genotype frequencies were found to be in HWE among the HC group (χ2 = 7.47, P < 0.01), whereas a deviation of the genotype frequencies from HWE was observed in the T2D and DN groups (χ2 = 0.17, P = 0.67; χ2 = 3.57, P = 0.06, respectively). When the subjects were categorized according to dyslipidemia, significant difference with respect to the genotype frequency was observed.
| Category (n) | Genotypes (%)* | Allele frequency | |||
|---|---|---|---|---|---|
| DD | ID | II | D | I | |
| HC (200) | 36 (18) | 75 (37.5) | 89 (44.5) | 0.37 | 0.63 |
| T2D (200) | 50 (25) | 97 (48.5) | 53 (26.5) | 0.49 | 0.51 |
| DN (200) | 85 (42.5) | 81 (40.5) | 34 (17) | 0.62 | 0.38 |
| Dyslipidemia | |||||
| T2D (73) | 27 (36.9) | 40 (54.8) | 6 (8.2) | 0.64 | 0.36 |
| DN (125) | 64 (51.2) | 48 (38.4) | 13 (10.4) | 0.7 | 0.3 |
| Non dyslipidemia | |||||
| T2D (127) | 23 (18.1) | 57 (43.2) | 47 (37.6) | 0.4 | 0.6 |
| DN (75) | 21 (28.0) | 33 (44.0) | 21 (28.0) | 0.50 | 0.5 |
*χ2P value. 1) DN vs. T2D=14.66;< 0.05. 2) Patients vs. HC=36.68;<0.05. 3) T2DM vs. HC=14.22;<0.05. χ2P value for dyslipidemia and non-dyslipidemia group. 4) DN vs.T2D=5.04;<0.05. 5) DN vs.T2D=3.26;>0.05
Unadjusted OR after the analysis of association as shown in the Table 3 revealed that among the patients (T2D and DN) vs. control group the OR for DD genotype was 2.29, (95% CI = 1.51–3.47; P < 0.001), whereas the OR for II genotype was 0.35, (95% CI = 0.24–0.50; P < 0.001). However, in T2D vs. HC group ID genotype, the OR was 1.56, (95% CI = 1.05–2.33; P = 0.03), whereas the risk for II was 0.44, (95% CI = 0.29–0.68; P < 0.001). When comparison was made among the disease population (i.e., DN vs. T2D) DD genotype showed risk of 2.217, (95% CI = 1.27–2.27; P < 0.001) toward DN and the OR for II genotype was 0.34, (95% CI = 0.19–0.59; P = 0.03). In the dyslipidemia, subgroup ID genotype gave a significant OR of 0.5 (95% CI = 0.28–0.92; P = 0.02). There was no significant association (P > 0.05) of the genotype with DN observed in the non-dyslipidemia subgroup.
| Category | Comparison of groups | Unadjusted OR (95% CI) | P |
|---|---|---|---|
| Patients vs. Controls | DD vs. ID+II | 2.32 (1.53-3.51) | <0.001 |
| ID vs. II+DD | 1.36 (0.94-1.89) | 0.37 | |
| II vs. ID+DD | 0.35 (0.24-0.50) | <0.001 | |
| T2D vs. HC | DD vs. ID+II | 1.51 (0.93-2.45) | 0.11 |
| ID vs. II+DD | 1.56 (1.05-2.33) | 0.03 | |
| II vs. ID+DD | 0.44 (0.29-0.68) | <0.001 | |
| DN vs. T2D | DD vs. ID+II | 2.22 (1.44-3.39) | <0.001 |
| ID vs. II+DD | 0.70 (0.48-1.07) | 0.10 | |
| II vs. ID+DD | 0.56 (0.34-0.92) | 0.03 | |
| Dyslipidemia DN vs. T2D | DD vs. ID+II | 1.78 (0.99-3.22) | 0.06 |
| ID vs. II+DD | 0.5 (0.28-0.92) | 0.02 | |
| II vs. ID+DD | 1.29 (0.47-3.57) | 0.63 | |
| Non dyslipidemia DN vs. T2D | DD vs. ID+II | 1.78 (0.89-3.45) | 0.11 |
| ID vs. II+DD | 0.96 (0.54-1.71) | 1 | |
| II vs. ID+DD | 0.66 (0.35-1.22) | 0.21 |
P<0.05 is considered to be significant
Further, we analyzed the risk of DN and T2D after adjusting with confounding variables such as age, sex, BMI, and lipid profile [Table 4]. DD genotype confers a risk of 2.6-fold (95% CI = 1.76–6.12; P = 0.019) toward progression to DN under the recessive genetic model. In addition, the II genotype seems to give a protective effect under the co-dominant model. In the dyslipidemia group, it was observed that II genotype and ID genotype showed protection under co-dominant (OR-0.05; 95% CI = 0.01–0.55; P = 0.01) and dominant model (OR-0.12; 95% CI = 0.01–1.03; P = 0.03).
| Model | DN vs. T2D | DN vs. HC | T2D vs. HC | Dyslipidemia DN vs. T2D | Non dyslipidemia DN vs. T2D | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | P | OR | P | OR | P | OR | P | OR | P | |
| Co-Dominant | ||||||||||
| I/I | 1.00 | <0.001 | 1.00 | 0.78 | 1.00 | 0.63 | 1.00 | 0.01* | 1.00 | 0.33 |
| I/D | 0.30 (0.10-0.91) | 1.45 (0.31-6.83) | 1.52 (0.58-3.97) | 0.05 (0.01-0.55) | 2.90 (0.49-17.12) | |||||
| D/D | 1.26 (0.43-3.68) | 1.91 (0.31-11.77) | 0.96 (0.24-3.83) | 0.30 (0.03-3.06) | 3.33 (0.53-21.06) | |||||
| Dominant | ||||||||||
| I/I | 1.00 | 0.34 | 1.00 | 0.54 | 1.00 | 0.49 | 1.00 | 0.03* | 1.00 | 0.14 |
| I/D-D/D | 0.63 (0.24-1.64) | 1.58 (0.36-6.85) | 1.38 (0.55-3.44) | 0.12 (0.01-1.03) | 3.09 (0.67-14.37) | |||||
| Recessive | ||||||||||
| I/I-I/D | 1.00 | 0.019 | 1.00 | 0.6 | 1.00 | 0.65 | 1.00 | 0.29 | 1.00 | 0.38 |
| D/D | 2.67 (1.17-6.12) | 1.47 (0.34-6.29) | 0.75 (0.21-2.62) | 2.23 (0.50-9.96) | 2.05 (0.40-10.49) | |||||
P<0.05 is considered to be significant
With regard to dyslipidemia status 10% in HC, 36.5% in T2D and 62.5% in DN dyslipidemia were observed. Comparison of lipid profile (TG, HDL, and LDL) between patients and HC group and DN and T2D group with respect to genotypes exhibited a variation in lipid levels (TG, HDL, and LDL), which was significant among DN and T2D group (P < 0.05) but not between HC and patients group though there was a trend (P > 0.05) [Figure 2].
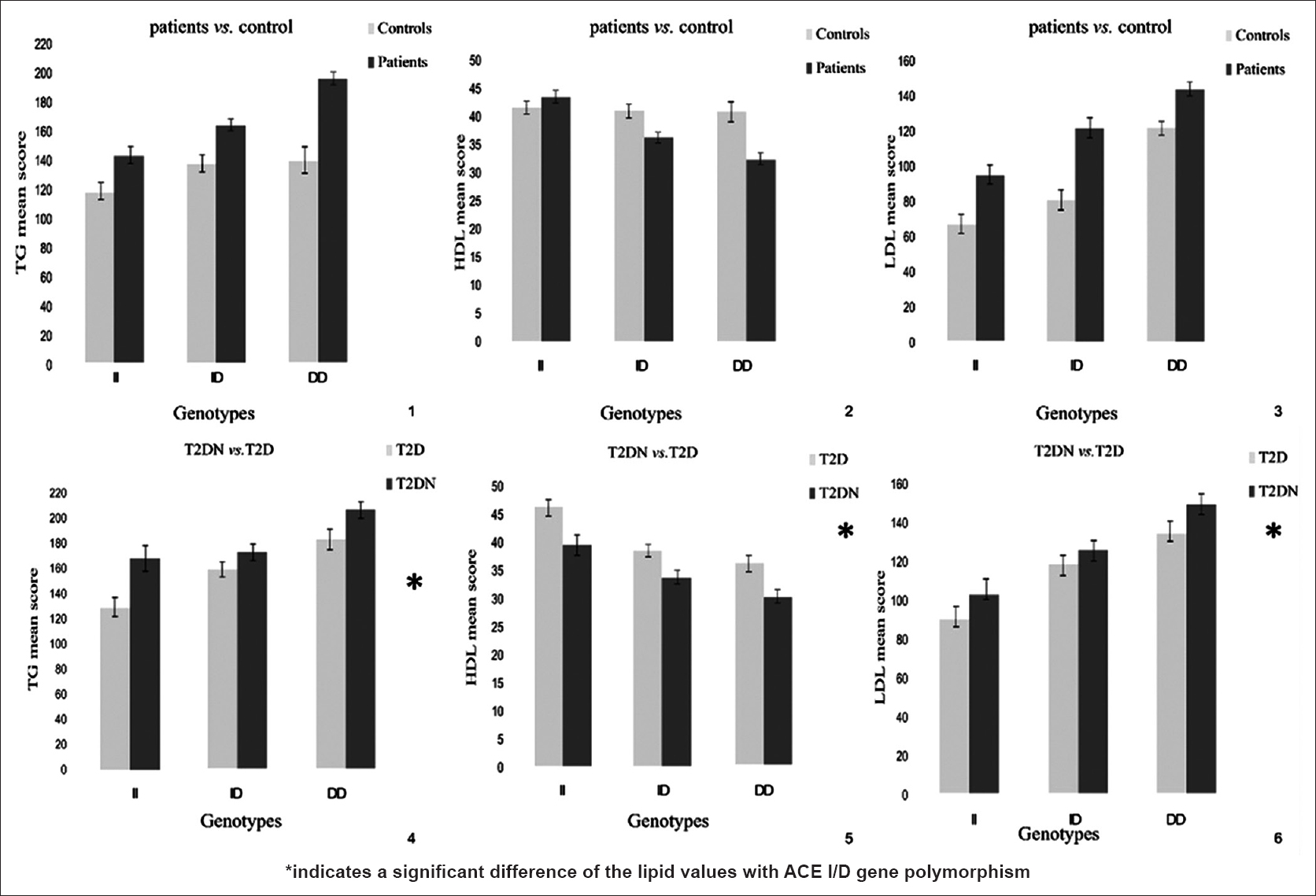
- Association of ACE I/D polymorphism with lipid profile (TG, HDL and LDL) between patients verses control and T2D verses T2DN group
The multiple logistic regression analysis was performed to look for the independent risk factors for DN among DN vs.T2D group. Male gender, BMI, hbA1c, TG, HDL, and ACE DD genotype emerged as independent risk factors for the development of DN [Table 5].
| Variable | OR (95% CI) | P |
|---|---|---|
| Age | 0.99 (0.97-1.02) | 0.91 |
| Male gender | 2.17 (1.39-3.39) | <0.001 |
| BMI | 1.11 (1.02-1.20) | 0.01 |
| hbA1c | 1.78 (1.4-2.24) | <0.001 |
| TG | 1.00 (0.99-1.00) | 0.05 |
| HDL | 0.95 (0.93-0.97) | <0.001 |
| LDL | 0.99 (0.98-1.00) | 0.13 |
| DD genotype | 1.96 (1.25-3.10) | <0.001 |
P<0.05 is considered to be significant
Discussion
DN, a multifactorial condition, is a major contributor to morbidity and mortality in patients with diabetes. Emerging evidence points toward the role of dyslipidemia as a crucial attendant risk factor in the pathogenesis of DN.[9] Moreover, ACE, a key component of the RAS pathway has been shown to be associated with dyslipidemia in a hyperglycemic milieu.[22] Although ACE gene variants and circulating levels have earlier been studied in relation to oxidative stress and diabetic complications, reports exploring the influence of genetic variants of ACE on serum lipids in DN are sparse. Given this background, the aims of the present study were of two-folds: (1) to see the relationship between ACE I/D gene polymorphism, a functional variant, which influences the expression levels of ACE in the occurrence of T2D and DN (2) to assess the ACE genotype-dependent effect on the serum lipids, in T2D and DN subjects.
In this study, we observed that the genotype and allele frequencies of the ACE I/D gene polymorphism differed significantly among the study groups. In addition, we observed that the presence of one copy of I allele (or the heterozygous genotype ID) offered a significant protective effect irrespective of the confounding variables, whereas the presence of two copies of “D” allele (as in DD genotype) conferred an almost three-fold risk for DN. Our observations are in line with an earlier report from India and elsewhere.[142324] However, Sikdar et al., (2013) found no association of ACE I/D polymorphism with DN.[25] This discrepancy between the previous study and ours might be owing to their relatively smaller sample size. Further, the report of Marre et al. on this polymorphism revealed a protective effect of II genotype in the development of DN.[26] Subsequently, a meta-analysis signified the association of D allele as a risk factor in the pathophysiology of DN.[27] Few studies showed lack of D allele association with DN and suggested a possible role of ACE-D allele in the progression rather than susceptibility to DN.[282930] The incongruities reported by various studies in the distribution of ACE I/D genotype could be owing to ethnic variation.[28] The protective role of II genotype in the development of DN was most pronounced in Asians with T2D followed by Caucasians with type 1 diabetes and T2D.[31]
In addition, in the risk analysis of DN and T2D according to ACE I/D genotypes under various genetic models, we detected that the significance of the ACE I/D polymorphism association with respect to the patients vs. controls and T2D vs. HC comparisons disappeared after adjustment for the confounding factors such as age, sex, dyslipidemia, and BMI. The result probably signifies that various factors interact with the specified genotypes in precipitating the diabetic phenotype. However, in the comparative group T2D vs. DN, the significance of genotype association persisted even after adjustment for the confounding variables. It can be surmised that for the predisposition from normoglycemia to hyperglycemia, ACE genotypes interact with the various risk factors, whereas in the setting of hyperglycemia, genotype seems a principal likely contributor to progression into complications such as DN. Another interesting feature is that the I allele seemed to confer a protective effect (co-dominant model), whereas two copies of D allele was found to be risky (recessive model).
The impact of ACE I/D polymorphism in T2D and DN could be eluded to the effect of the polymorphic variant at the molecular level on the expression of the ACE gene. Published data indicate that the D allele of the ACE I/D polymorphism leads to constitutive high level of ACE, which is deleterious to the kidney in a hyperglycemic milieu, whereas the I allele leads to relatively lower plasma ACE levels, there by exhibiting a protective effect against the disease. The DD genotype of ACE gene is strongly associated with the increased serum ACE activity that may predispose the diabetic individuals to complications.[24] Existing evidence points toward a probable link between plasma lipoprotein and angiotensin II induced-renal injury of DN. Elevated angiotensin II levels (owing to elevated systemic/localized ACE levels) may promote oxidative stress favoring the formation of oxidized LDL a key mediator in the cell injury resulting in the formation of renal lesions. As a result, glomerular capillary hypertension is built-up, thereby enhancing glomerular permeability of macromolecules leading to both mesangium lipid accumulation and tubular lipoprotein overload. Finally, angiotensin II stimulates the release of chemokines, cytokines, potentially enhancing infiltration, and accumulations of lipids into macrophage that causes extracellular matrix accumulation further promoting to glomerular and tubular-interstitial injury.[8]
Thus, although ACE is supposedly a crucial factor in predisposition to DN through its causation of oxidative stress by the formation of ox-LDL, dyslipidemia attendant with hyperglycemia has been suggested to be another key factor in the pathogenesis of DN.[9] ACE I/D genotypes and plasma ACE levels have been reported to associate with dyslipidemia.[18] Fairly large number of studies exist regarding ACE and its association with DN, but there are barely any reports pertaining to the analysis of ACE genotypes and DN subjects categorized on the basis of dyslipidemic status. Interestingly, we observed a significant protective effect of ACE I/D genotype with DN (co-dominant model) in dyslipidemic subjects but not within the non-dyslipidemia group. The result might signify the mildly protective contribution of the “I” allele in a dyslipidemic scenario as opposed to the deleterious influence of the “D” allele. An earlier study on Taiwanese population reported the association of atherosclerotic risk factors such as hypertension, smoking, dyslipidemia, and obesity with ACE genotypes in T2D patients with albuminuria.[9] It is plausible that the ACE I/D polymorphism through its effect on circulating ACE levels might be a key contributor to dyslipidemia in a hyperglycemic environment further culminating in renal complications. However, there was significant association of ACE I/D polymorphism noted in the non-dyslipidemic subjects. DN being a multifactorial condition, the presence or absence of predisposing genotypes at other relevant loci could influence the phenotype, warranting the association study of further putative genetic markers with DN in this subgroup of patients.
Moreover, in the present study, we sought to see if there is any interaction between ACE genotypes and lipid levels. We found a genotype-dependent variation in the plasma TG, HDL, and LDL levels among the various study groups. Importantly, the DD genotype subjects in the DN subgroup were characterized by a significantly unhealthy lipid profile. Nagi et al. (1998) found that plasma ACE levels were associated with plasma triglycerides and total cholesterol levels in diabetic subjects among Pima Indians.[32] This further strengthens our premise that ACE DD genotype is a plausible factor responsible for dyslipidemia.
Besides, we also sought to explore the independent effect of potential risk factors for DN employing MLR. In corroboration with our results presented earlier, we observed the DD genotype, TG, and HDL emerged as independent risk factors for progression into DN. However, LDL did not show any significant association. This could be explained by the view that diabetic dyslipidemia is characterized by an elevated serum TG and a reduced HDL-cholesterol without marked alteration in LDL-cholesterol.[81122] In addition, BMI was found to be significant independent predictor for DN. It is interesting here to note that weight loss through lifestyle modifications has been reported to reduce proteinuria in DN.[33] Further, male gender and HbA1c also appeared as a significant risk predictors for DN in MLR. Glycated hemoglobin is a potent indicator of long-term glycemic control signifying that effective modulation of glucose levels is essential in the progression into diabetic complications, especially DN. In fact, variability in HbA1c was previously reported to correlate independently with DN.[3435] Association with male gender that we observed in this study could possibly be the outcome of hormonal influences and must be interpreted cautiously considering that conflicting associations have been reported previously.
On the basis of all these results, it can be carefully inferred that ACE I/D polymorphism and dyslipidemia lead into DN both independently as well as through a complex interaction. It would be interesting to analyze these allelic interactions at the cellular functional level so as to get an insight into the operative effect of ACE I/D variant in the alteration of lipid balance and leading to the causation of DN. Analysis of the pharmacogenetic response of ACE inhibitors would add additional information to design effective patient care strategies.
Conclusion
The study showed a significant predisposing association of ACE DD genotype with DN and protective effect of ID genotype on DN in the dyslipidemia sub group. Furthermore, serum lipids varied significantly with respect to the ACE genotypes suggesting an interaction between the genotypes and concomitant TG, HDL, and LDL levels.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank all the study subjects for their will full participation in this study. I deeply thank late Dr. Mohammed Siraj Physician and Diabetologist for providing me the samples.
References
- Diabetes mellitus – A devastating metabolic disorder. Asian J Biomed Pharm Sci. 2014;4:1-7.
- [Google Scholar]
- Diabetes in Asia. Epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129-40.
- [Google Scholar]
- Abdominal fat: Does it predict the development of type 2 diabetes? Am J Clin Nutr. 2008;87:1118-9.
- [Google Scholar]
- Cell biology of diabetic nephropathy: Roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Invest. 2015;6:3-15.
- [Google Scholar]
- Current challenges in diabetic nephropathy: Early diagnosis and ways to improve outcomes. Endocrinol Metab. 2016;31:245-53.
- [Google Scholar]
- A preliminary study on NPHS2 Gene Polymorphism (R229Q) in Diabetic Nephropathy patients from South India. J Cell Sci Molecul Biol. 2014;1:108.
- [Google Scholar]
- Association of angiotensin converting enzyme insertion-deletion polymorphism with hypertension in emiratis with type 2 diabetes mellitus and its interaction with obesity status. Dis Markers. 2015;2015:536041.
- [Google Scholar]
- Joint effects of hypertension, smoking, dyslipidemia and obesity and angiotensin-converting enzyme DD genotype on albuminuria in Taiwanese patients with type 2 diabetes mellitus. Clin Biochem. 2010;43:629-34.
- [Google Scholar]
- Role of the renin angiotensin system in diabetic nephropathy. World J Diabetes. 2010;1:141-5.
- [Google Scholar]
- ACE insertion/deletion (I/D) polymorphism and diabetic nephropathy. J Nephropathology. 2012;1:143-51.
- [Google Scholar]
- Angiotensin converting enzyme (ACE) gene polymorphism increases the susceptibility of diabetic nephropathy in Western Indian Type 2 diabetic patients. Int J Diab Dev Ctries. 2011;31:223-9.
- [Google Scholar]
- An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Inves. 1990;86:1343-6.
- [Google Scholar]
- Variations in angiotensin-converting enzyme gene insertion/deletion polymorphism in Indian populations of different ethnic origins. J Biosci. 2002;27:67-70.
- [Google Scholar]
- ACE gene insertion/deletion polymorphism associated with 1998 World Health Organization definition of metabolic syndrome in Chinese type 2 diabetic patients. Diabetes Care. 2002;25:1002-8.
- [Google Scholar]
- Cut off values for normal anthropometric variables in Asian Indian Adult. Diabetes Care. 2003;26:1380-4.
- [Google Scholar]
- Prevalence of Dyslipidemia in Urban and Rural India: The ICMR–INDIAB study. PLoS One. 2014;9:e96808.
- [Google Scholar]
- Does disease status and steroid responsiveness in idiopathic nephrotic syndrome depends on ACE I/D polymorphism? A study from South India. Asian J Biol Sci. 2012;5:148-56.
- [Google Scholar]
- Association of angiotensin I converting enzyme, angiotensin II type 1 receptor and angiotensin I converting enzyme 2 gene polymorphisms with the dyslipidemia in Type 2 diabetic patients of Chinese Han origin. J Endocrinol Invest. 2012;35:378-83.
- [Google Scholar]
- Relationship of angiotensin-converting enzyme gene polymorphism with nephropathy associated with Type 2 diabete smellitusin Asian Indians. J Diabetes Complications. 2007;21:237-41.
- [Google Scholar]
- Association between ACE gene polymorphism and diabetic nephropathy in South Indian Patients. JOP. 2001;2:83-7.
- [Google Scholar]
- ACE gene insertion/deletion polymorphism and type-2 diabetic nephropathy in Eastern Indian population. Human Biol Rev. 2013;2:66-76.
- [Google Scholar]
- Relationships between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complications. Diabetes. 1994;43:384-88.
- [Google Scholar]
- Association between the ACEI/D gene polymorphism and DN susceptibility: The risk of T2DM developing into DN in the Asian population. J Renin Angiotensin Aldosterone Syst. 2015;16:36-44.
- [Google Scholar]
- Angiotensin-I converting enzyme gene polymorphism in Turkish type 2 diabetic patients. Exp Mol Med. 2004;36:345-50.
- [Google Scholar]
- angiotensin-iconvrting enzyme polymorphism and diabetic nephropathy in North India. Int J Hum Genet. 2005;5:279-83.
- [Google Scholar]
- Effect of angiotensin convertingenzyme (ACE) genepolymorphismon progression of renal disease and the influence of ACE inhibition in diabetic patients. Diabetes. 1998;47:1507-11.
- [Google Scholar]
- Angiotensin I converting enzyme insertion/deletion polymorphism and its association with diabetic nephropathy: A meta-analysis of studies reported between 1994 and 2004 and comprising 14,727 subjects. Diabetologia. 2005;48:1008-16.
- [Google Scholar]
- Angiotensin-1-converting enzyme (ACE) gene polymorphism, plasma ACE levels, and their association with the metabolic syndrome and electrocardiographic coronary artery disease in Pima Indian. Metabolism. 1998;47:622-6.
- [Google Scholar]
- Weight loss for reduction of proteinuria in diabetic nephropathy: Comparison with angiotensin-converting enzyme inhibitor therapy. Indian J Nephrol. 2013;23:108-13.
- [Google Scholar]
- HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: The renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care. 2013;36:2301-10.
- [Google Scholar]
- Fluctuations in glycosylated hemoglobin (HbA1C) as a predictor for the development of diabetic nephropathy in type 1 diabetic patients. Int J Diabetes Mellitus. 2010;2:10-14.
- [Google Scholar]







