Translate this page into:
Lymphomatous Interstitial Nephritis Coexistent with Paraneoplastic Crescentic Membranoproliferative Glomerulonephritis in a Case of Mantle Cell Lymphoma
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Mantle cell lymphoma (MCL) is a rare aggressive lymphoproliferative disorders (LPD) of B-cell lymphoma, which usually presents in advanced stages at initial diagnosis. Renal involvement in MCL is very rare, especially the combined presence of both glomerular and interstitial disease. We report on a patient with lymphomatous interstitial nephritis (LIN) coexistent with paraneoplastic crescentic membranoproliferative glomerulonephritis (MPGN), subsequently diagnosed to have disseminated MCL with bone marrow and lymph nodal infiltration. He was treated with rituximab-based chemotherapy and went into complete renal remission at 6-months of follow up.
Keywords
Crescentic membranoproliferative glomerulonephritis
lymphomatous interstitial nephritis
mantle cell lymphoma
paraneoplastic glomerulonephritis
renal lymphoma
Introduction
Mantle cell lymphoma (MCL) is a rare aggressive form of B-cell non-Hodgkin lymphoma (NHL), representing 3–10% of all NHLs. It results from malignant transformation of B lymphocytes in the outer edge of a lymph node follicle called the mantle cell zone and is characterized by proliferation of lymphocytes that infiltrate lymphoid tissues, bone marrow, peripheral blood, and extranodal sites.[1] Renal involvement in MCL is very rare. There are only a few isolated reports of paraneoplastic glomerulonephritis (p-GN) and lymphomatous interstitial nephritis (LIN) due to MCL. But combined presence of both glomerular and interstitial disease is extremely rare. Till date, only two cases of renal interstitial infiltration by MCL coexistent with crescentic membranoproliferative glomerulonephritis (MPGN) have been described. Here we present a patient with suspected glomerular disease with a few extrarenal features, subsequently diagnosed to have disseminated MCL, having combined p-GN and LIN on renal biopsy.
Case History
A 58-year-old man with no prior comorbidities presented with three weeks history of malaise, pedal edema and oliguria. It was not associated with cola colored urine, skin rash, fever, pain killers, and native medicine usage. There was no significant weight loss and bony pains. On examination, he had high blood pressure (144/90 mm Hg) with pallor, pitting pedal edema, generalized lymphadenopathy and mild splenomegaly. Ocular fundus examination and rest of the systemic examination were unremarkable.
The laboratory findings revealed hemoglobin of 9.0 g/dl, total leukocyte count of 12.0 × 103 cells/μL with differential count of polymorphs - 44% and lymphocytes - 40%, platelet count of 1.6 × 105/μL, serum creatinine of 2.6 mg/dl, and serum albumin of 2.7 g/dl. Dyslipidemia was also noted. His urine examination showed 3+ albumin, 12–14 erythrocytes/hpf and 10–12 pus cells/hpf. Spot urine protein to creatinine ratio was 3.8. Serology for hepatitis B and C, human immunodeficiency virus (HIV), anti-nuclear antibody (ANA) and anti-neutrophil cytoplasmic antibody (ANCA) was negative. Serum complement levels and anti-streptolysin O (ASO) titer were normal. Urine culture did not show any bacterial growth. Ultrasonography showed mildly bulky kidneys (10.3 and 10.8 cm length of right and left kidneys respectively). As he was having significant extrarenal features, we suspected a glomerular disease secondary to either infection, autoimmune, or neoplastic etiology, hence renal biopsy was performed.
In renal biopsy, on light microscopy, 4 out of 14 glomeruli were obsolescent and another 6 glomeruli showed crescents (4 fibrocellular and 2 cellular). The underlying glomerular tufts showed variable mesangial and endocapillary proliferation, and irregular basement membrane thickening with focal duplication. The interstitium was widened with dense nodular infiltrate of lymphocytes, which were monomorphic histologically. Interstitial fibrosis and tubular atrophy (IF/TA) was up to 30% of the cortex sampled; blood vessels appeared normal. Immunofluorescence (six glomeruli) was positive for significant peripheral and mesangial granular deposits of immunoglobulins IgG (3+), IgM (2+) and complements C3c (3+) and C1q (3+) [Figures 1 and 2]. As interstitial lymphoid infiltrate was dense and fairly monomorphic. On immunohistochemistry (IHC) CD5, CD20, Cyclin D1, Bcl-2 were positive in lymphoid aggregates and CD10 was negative [Figure 3]. The immune profile was consistent with MCL. Electron microscopy was not done. Kidney biopsy showed features of combined immune complex mediated crescentic MPGN and interstitial infiltration by MCL. Further evaluation was performed to exclude a systemic lymphoma. Serum uric acid and lactate dehydrogenase levels were elevated; serum calcium was normal. Bone marrow biopsy showed lymphocytosis with nodular replacement of intertrabecular spaces by small sized atypical lymphoid cells with relatively suppressed hematopoiesis; nuclei were focally convoluted with less condensed nuclear chromatin. Lymph node biopsy was also suggestive of involvement by MCL. Patient was diagnosed with stage 4 MCL with LIN and coexistent crescentic MPGN. He was treated with cytotoxic chemotherapy in combination with rituximab and showed a dramatic therapeutic response leading to complete renal remission at 6-months of follow up.

- Renal biopsy showing patchy dense nodular lymphoid infiltrate in the interstitium (Panel a - periodic acid-Schiff (PAS), ×40). High power view of the infiltrate showing monomorphic lymphoid cells (Panel b - hematoxylin-eosin, ×400)
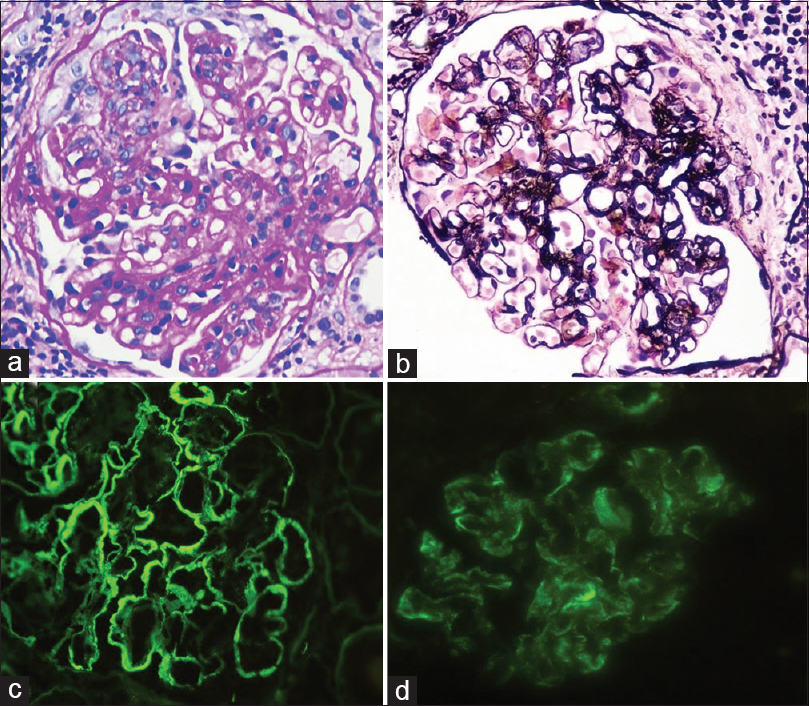
- Glomerulus showing an MPGN pattern glomerulonephritis with mesangial and endocapillary proliferation and segmental duplication of the basement membrane (Panel a – PAS, ×400; Panel b - Jones silver, ×400). Direct immunofluorescence stains showing significant peripheral deposits of IgG (Panel c, ×400) and C3c (Panel d, ×400)
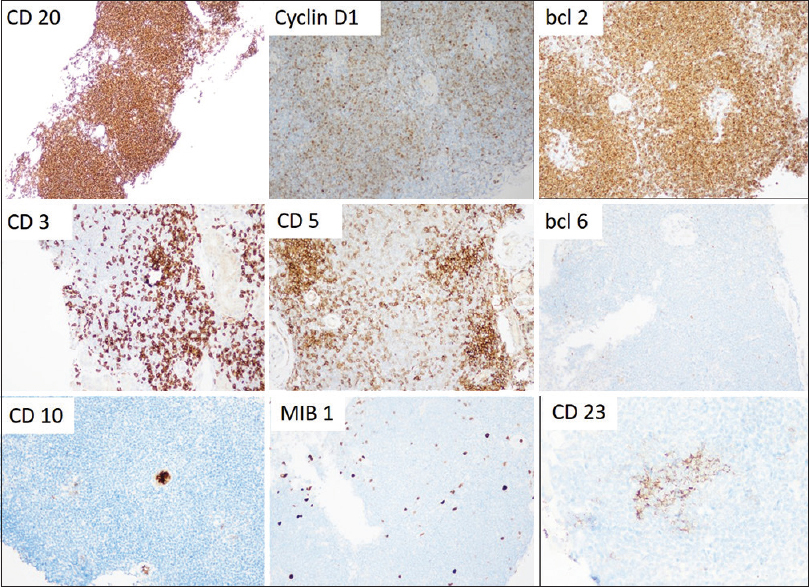
- Composite image of the panel of immunohistochemical markers of the lymphoid infiltrate. There is diffuse strong positivity for CD20, cyclin D1, and BCL-2. The intervening small lymphocytes are CD3 and CD5 positive. The MIB1 index is low averaging 5%. CD10, BCL-6, and CD23 are essentially negative
Discussion
MCL is a rare form of NHL having a reported incidence of 0.5–2 per 100,000 individuals. The median age at diagnosis is between 60 and 70 years. The disease is 2-4 times more common in men than in women. It is characterized genetically by the chromosomal translocation t(11;14) (q13;q32), which is found to be present in most cases of MCL and leads to over expression of cyclin D1 and deregulation of the cell cycle. It usually presents very late in its disease course with most of the patients present with disseminated disease (stage 3 or 4) at the time of diagnosis. The most common extranodal sites of involvement are bone marrow, liver, spleen, gastrointestinal tract and Waldeyer's ring. The renal involvement in MCL is seldom reported.
The various causes of renal involvement in a lymphoma are tumor lysis syndrome, hypercalcemia, hyperuricemia, hemolysis, paraproteinemia, cryoglobulinemia, amyloidosis, p-GN, radiotherapy/chemotherapy-related effects, direct infiltration of kidneys (LIN), extrinsic compression of urinary tract and renal vessels, and development of primary renal lymphoma.[23] P-GN seems to be more common in Hodgkin's than in NHL.[3] LIN may occur in 6–60% of NHLs, depending on its subtype. Clinically, renal involvement in lymphoma can present with acute kidney injury (AKI), proteinuria, hematuria, nephrotic syndrome (NS), hypertension, and chronic kidney disease (CKD). The various forms of p-GN reported in association with lymphomas were MPGN, membranous GN (MGN), minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), crescentic GN, mesangioproliferative GN, IgA nephropathy, amyloidosis, monoclonal immunoglobulin deposition disease (MIDD), fibrillary GN and immunotactoid glomerulopathy.[4] A retrospective study of renal manifestations in 700 patients with documented lymphoma revealed MPGN as the most common glomerular lesion, followed by MGN.[4]
So far, 20 cases of pathologically proven renal involvement due to MCL have been described in the literature. 12 cases of p-GN in the setting of MCL have been described.[3456789101112] LIN due to MCL has been observed in 4 cases.[13141516] The coexistence of LIN and p-GN due to MCL is extremely rare. Till date, only 4 of these cases have been reported globally [Table 1].[17181920] Interestingly, all of them have presented with crescentic GN and LIN; of these four cases, two were having crescentic MPGN.[1920] Our patient was also diagnosed to have crescentic MPGN and LIN. In most of these cases, renal disease was coexisted or followed the diagnosis of MCL. Very few reports have noted renal disease as the first manifestation of MCL, with the observed latency between renal disease and MCL diagnosis of up to 6 years.[9] A few others described primary renal MCL limited to kidneys alone.[16]
| Case | Age/Sex | Renal biopsy | Renal presentation | Order of presentation | Renal outcome | References |
|---|---|---|---|---|---|---|
| 1 | 46/F | ANCA-negative pauci-immune crescentic GN + LIN | RPRF + proteinuria | Simultaneous | Complete recovery | Wang J, et al. |
| 2 | 54/M | Mesangial proliferation with crescents + LIN | AKI + proteinuria | Simultaneous | Partial recovery | Peddi S, et al. |
| 3 | 65/M | Crescentic MPGN+LIN | AKI + nephritic syndrome | Simultaneous | Complete recovery | Montoro J, et al. |
| 4 | 77/M | Crescentic MPGN+LIN | AKI + proteinuria | Simultaneous | Complete recovery | Lubas A, et al. |
| 5 | 58/M | Crescentic MPGN+LIN | AKI + proteinuria | Simultaneous | Complete recovery | Our case |
ANCA: Anti-neutrophil cytoplasmic antibody, GN: Glomerulonephritis, LIN: Lymphomatous interstitial nephritis, RPRF: Rapidly progressive renal failure, AKI: Acute kidney injury, MPGN: Membranoproliferative glomerulonephritis, M: Male, F: Female
MCL is typically diagnosed by tissue biopsy of a lymph node, tissue, or bone marrow demonstrating the classical cytologic appearance of a monomorphic proliferation of small to medium lymphoid cells with irregular or cleaved nuclei, condensed chromatin, indistinct nucleoli and scant cytoplasm forming diffuse, nodular or mantle zone patterns. On IHC staining, it typically expresses B cell antigens (CD19, CD20, CD22), CD5, cyclin D1, and is negative for CD10, CD23, and BCL-6, which helps to establish diagnosis and differentiate MCL from other NHL subtypes. BCL-2 is overexpressed in indolent B-cell NHLs including MCL. The Ki-67 proliferative index using the monoclonal antibody MIB1 is a marker of cell proliferation rate and a prognostic factor. The diagnosis of MCL is confirmed with immunohistochemistry (IHC), or with detection of the t (11;14) (q13;q32) translocation by molecular or cytogenetic methods.
In our patient, immune complex mediated crescentic MPGN is probably paraneoplastic in origin due to deposition of immune complexes containing tumor antigens. The temporal association between renal involvement and lymphoma disease activity, and the improvement in renal function following cytotoxic therapy would be consistent with p-GN. Our patient was having low MIB1 index.
Although there is no standard of care for MCL, it is usually treated with combination chemotherapy plus rituximab, followed by autologous stem cell transplantation especially in young patients. It is associated with poor prognosis with high relapse rates. Despite improvement in overall prognosis with currently available therapies, relapses are still common in MCL. In most of the reported cases, good renal recovery was noted after chemotherapy. Our patient was treated with rituximab-based chemotherapy and went into complete renal remission at 6-months of followup.
High index of suspicion and screening to rule out underlying malignancy is essential in the management of glomerular disorders. Prominent interstitial lymphoid cell infiltration in a case of GN gives a clue to underlying LPD as in our case. Early diagnosis and prompt chemotherapy result in good renal recovery as noted in most of the reported cases, including ours.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019-32.
- [Google Scholar]
- Acute renal failure due to a malignant lymphoma infiltration uncovered by renal biopsy. Nephrol Dial Transplant. 2004;19:2657-60.
- [Google Scholar]
- Crescentic glomerulonephritis and centrocytic lymphoma. Nephrol Dial Transplant. 1999;14:1744-5.
- [Google Scholar]
- Kidney involvement and renal manifestations in non-Hodgkin's lymphoma and lymphocytic leukemia: A retrospective study in 700 patients. Eur J Haematol. 2001;67:158-64.
- [Google Scholar]
- Proliferative glomerulonephritis associated with mantle cell lymphoma - natural history and effect of treatment in 2 cases. Clin Nephrol. 2004;61:422-8.
- [Google Scholar]
- Antineutrophil cytoplasmic antibody-positive pauci-immune glomerulonephritis associated with mantle cell lymphoma. Clin Nephrol Case Stud. 2017;5:9-15.
- [Google Scholar]
- An 80-year-old man with renal insufficiency, proteinuria, hematuria, hemiparesis, and pleuritis. Am J Kidney Dis. 2004;44:1121-5.
- [Google Scholar]
- Association of membranoproliferative glomerulonephritis with mantle cell lymphoma. BMJ Case Reports 2013 doi: 101136/bcr-2013-009730
- [Google Scholar]
- Mantle cell lymphoma associated with membranoproliferative and membranous glomerulonephritis: Report of two cases. Cancer Sci Res Open Access. 2016;3:1-7.
- [Google Scholar]
- Minimal change disease associated with newly diagnosed mantle cell lymphoma. Renal Failure. 2014;36:634-7.
- [Google Scholar]
- Successful treatment of focal segmental glomerulosclerosis in association with mantle cell lymphoma. Ren Fail. 2007;29:363-6.
- [Google Scholar]
- Mantle cell lymphoma first presenting as immune complex-mediated glomerulonephritis: A case report. J Med Case Rep. 2015;9:115-8.
- [Google Scholar]
- Acute renal failure due to mantle cell lymphoma - A case report and discussion of the literature. Clin Nephrol. 2007;67:394-6.
- [Google Scholar]
- Renal involvement of mantle cell lymphoma leading to end stage renal disease. Hemodial Int. 2012;16:104-8.
- [Google Scholar]
- Acute tubulointerstitial nephritis associated with mantle cell lymphoma presented as acute renal failure. Nephrology. 2007;12:107-8.
- [Google Scholar]
- Primary pleomorphic mantle cell lymphoma of kidney: A case report. Int J Cancer Manag. 2018;11:e10203.
- [Google Scholar]
- Antineutrophil cytoplasmic antibody-negative pauci-immune crescentic glomerulonephritis and mantle-cell lymphoma. A case report and review of the literature. WIMJ Open. 2014;1:114-6.
- [Google Scholar]
- Acute renal failure in a patient with mantle cell lymphoma. Hemodial Int. 2015;19:E12-5.
- [Google Scholar]
- Acute renal failure due to membranoproliferative glomerulonephritis and kidney tumoral infiltration in a patient with mantle cell lymphoma. Hematol Transfus Int J. 2015;1:12-4.
- [Google Scholar]
- Membranoproliferative glomerulonephritis, mantle cell lymphoma infiltration, and acute kidney injury. Int Urol Nephrol. 2013;45:1489-94.
- [Google Scholar]







