Translate this page into:
Clinico-pathological Profile and Outcome of C-3 Glomerulopathy in Indian Children
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
There is paucity of data of C3 glomerulopathy in Indian children.
Methods:
First Indian pediatric case series where consecutive renal biopsies done over a period of ten years were reviewed to identify those patients who had isolated or predominant C3 deposits on immunofluorescent microscopy, fulfilling the criteria for C-3 glomerulopathy. The clinical, biochemical, serological, histopathological profile, eGFR and the need for renal replacement therapy was analyzed.
Results:
Eighteen patients, comprising 5.3% (18/298) of all renal biopsies, had C3 glomerulopathy, four with Dense Deposit Disease (DDD) and fourteen with C3 Glomerulonephritis (C3GN) with a median follow-up of 38.2 months. Median age of presentation was 7.45±3.03 years (2.5yrs- 13.5yrs) with nine boys and nine girls. Presentation was nephrotic syndrome in seven (39%), acute nephritic syndrome in three (16.7%), hematuria in five (27.7%) and acute kidney injury in three (16.7%). Median eGFR was 69 ml/min/1.73m2 (8.2-107 ml/min/1.73m2). Hematuria was seen in 16 (88%), proteinuria in 18 (100%) and low C3 in 16 (88%) at the time of presentation. Mesangioproliferative glomerulonephritis was the predominant pattern in DDD while C3GN showed a mix of mesangioproliferative, membranoproliferative, endocapillary and crescentic GN (p = 0.43).Complete or partial remission was seen in seven patients who received long term alternate day steroids alone or with added mycophenolate mofetil. The cumulative patient survival was 70.8%. Kaplan Meir analyses for renal survival without progression to ESRD was 60.2% at one year and 48.1% at five and ten years.
Conclusion:
Interstitial fibrosis and tubular atrophy on renal biopsy was an independent predictor of adverse renal outcome in the cohort (p = 0.013, HR8.1;95% CI -1.6-42).
Keywords
C3 glomerulonephritis
C3 glomerulopathy
dense deposit disease
end stage renal disease (ESRD)
Introduction
Isolated or dominant C3 deposits on immunofluorescent (IF) microscopy is the phenotype which identifies C3 glomerulopathy (C3GP) that occurs due to dysfunction of the alternate pathway of complement. Evaluation by serology and genetic testing can identify the etiopathogenesis of the alternate pathway dysfunction in many but not in all cases. Complement dysfunction can result from genetic defects leading to deficiencies of complement factors or complement regulatory proteins with low levels of C3, Factor B, Factor H, Factor I, or Membrane cofactor protein. It could also occur due to acquired antibodies to Factor H, Factor B, or C3 convertase (C3 nephritic factor). As facilities to investigate the complement factors are not easily available to clinicians, most cases of C3GN remain etiologically undefined. C3GN is currently broadly classified based on the pattern of electron dense deposits on electron microscopy as DDD with ribbon like intramembranous osmiophilic deposits or as C3GN with variable deposits other than dense intramembranous deposits.[12] Since its formal nomenclature in 2012[1] several case series have been published, largely of adults and adolescent children including two publications from India.[34] Ours is the first Indian pediatric case series describing the clinico-pathological profile and outcome of C3GN in 18 prepubertal children biopsied over a 10-year period.
Methodology
This is a retrospective case series of all children with C3GN seen over a period of 10 years in the Division of Pediatric Nephrology at a tertiary care children's hospital. All renal biopsies done at our Centre from January 1, 2007 to December 31, 2016 were reviewed. The immunofluorescent microscopy (IF) results were noted for the presence or absence of C3, IgG, IgM, IgA, and C1q. The intensity of the immunoreactants was graded on a scale of 0, trace, 1+, 2+, 3+.
Inclusion criteria
Patients included were all those whose renal biopsy on IF microscopy showed[2]:
-
Dominant C3 deposits with the intensity 2+/3+ with at least two orders of magnitude more intense than any other visible immunoreactant.
Exclusion criteria
-
Patients whose renal biopsies showed no glomeruli in the core sent for IF
-
Patients with post-infectious glomerulonephritis, with subepithelial humps on Electron Microscopy (EM) and C3 deposition on IF, who made full clinical and serologic recovery within 12 weeks of presentation including normalization of C3 levels.
The medical records of patients who met the inclusion criteria were reviewed. Demographic details, clinical features, and laboratory findings at initial presentation were noted. Details of the clinical course, treatment given, date and age at last follow-up, presence and severity of proteinuria, serum creatinine levels and the institution of dialysis were noted. Initial presentation was classified as nephrotic syndrome (NS), acute nephritis, hematuria, and acute kidney injury.
Light microscopy (LM) findings were reviewed for the underlying morphology, presence and number of crescents, glomerulosclerosis, interstitial fibrosis and tubular atrophy (IFTA). Electron microscopy (EM) findings were noted for the presence, location and extent of electron dense deposits (EDD). Patients were identified as DDD based on EM findings of extensive intramembranous ribbon like EDD. The outcome at last follow up was classified as death, ESRD with or without dialysis and survival without ESRD.
Clinical definitions used in the study:[56]
-
Hematuria—>5 RBCs per high power field on microscopic examination of the urinary sediment
-
Nephrotic range proteinuria—24-hour urine protein >40 mg/m2/h or ≥50 mg/kg/day or spot urine protein/creatinine >2 mg/mg in random urine sample;
-
Nephrotic syndrome (NS) —Nephrotic range proteinuria, hypoalbuminemia <31.5 g/dL, with or without edema
-
End Stage Renal Disease (ESRD) - eGFR <31 ml/min/1.73 m2 or dialysis dependent;
-
Complete remission (CR)- Absence of edema, serum albumin >3.5 g/dl, Reduction of proteinuria to <31.3 g/d or <31 mg/g, normal serum creatinine
-
Partial remission (PR) —Absence of edema with reduction in proteinuria to >0.3 g/day but less than the nephrotic range of proteinuria or with a decrease of at least 50% in the degree of proteinuria and stable or <31% change in serum creatinine.
Statistical analysis
Descriptive statistics for continuous variables was presented as mean +/- SD. Categorical variables were expressed as frequencies (%) with median values. Comparison of groups was done by Chi square test. Statistical analysis was performed using Microsoft excel for Windows. Survival estimates (end point = death, dialysis, or eGFR <31 ml/min/1.73 m2) were computed using Kaplan and Meir Survival analyses. Cox regression model was used to identify the variables affecting renal outcome and survival. Statistical significance was assumed at P < 0.05.
The study was approved by the Hospital's Institutional Ethics Committee Board.
Results
There were 298 biopsies done during the study period. Twenty-one biopsies were excluded from analysis as the core for IF showed no glomeruli. Of the remaining 277 biopsies, 41 had isolated or dominant C3 deposits on IF. 23/41 were excluded as they were classical postinfectious GN with complete recovery over 12 weeks [Figure 1].

- Flow chart depicting the methodology of selecting children for the study. IF, Immunofluorescence; PIGN, Post infectious glomerulonephritis
18 biopsies fulfilled the criteria for C3GN. This included 4 with DDD and 14 with C3GN. Seven biopsies were reclassified as C3GN. These included cases that were earlier classified as membranoproliferative glomerulonephritis (MPGN) in 4, mesangioproliferative glomerulonephritis (MesPGN) in 2 and crescentic glomerulonephritis (CGN) in 1 patient.
Clinicopathological profile of all patients
There were 9 boys and 9 girls with a mean age of 7.45 ± 3.03 years (range: 2.5-13.5 years) at onset [Table 1]. Presenting features were nephrotic syndrome (NS) in 7, hematuria in 5, acute nephritis in 3, and AKI in 3. Hypertension was found in 11 patients. The creatinine at presentation ranged from 0.3–8.4 mg/dL (Median: 0.7mg/dL) with a glomerular filtration rate ranging from 8.2–107 ml/min/1.73 m2 (median: 69 ml/min/1.73 m2). Proteinuria was present in all 18 patients with nephrotic range proteinuria in 14; mean urinary protein was 1575 ± 1day. Hematuria was seen in 16 children, gross in 12 and microscopic in 4. Serum C3 levels ranged from 13-142 mg/dL with a mean of 50.3 ± 37.5 mg/dl and was low in 16 patients (88%) [Table 1].
| Category | Dense deposit disease (n=4) | C3 Glomerulo nephritis (n=14) | Total (n=18) | P |
|---|---|---|---|---|
| Age (years) | ||||
| Mean±SD | 7.3±2.9 | 7.5±3.3 | 7.5±3.0 | 0.44 |
| Age Range | 4.0-11.0 | 2.5-13.5 | 2.5-13.5 | |
| Median (IQR) | 7 (4.5-10.3) | 8 (4.6-10.5) | 8 (4.6-10.5) | |
| Male Sex (n, %) | 3 (75.0) | 6 (42.8) | 9 (50.0) | 0.51 |
| Presenting Features (n, %) | ||||
| Nephrotic syndrome | 1 (25.0) | 6 (42.8) | 7 (38.8) | 0.26 |
| Nephritic syndrome | 2 (50.0) | 1 (7.1) | 3 (16.7) | 0.66 |
| Hematuria | 1 (25.0) | 4 (28.6) | 5 (28) | 0.72 |
| Acute kidney injury | 0 | 3 (21.4) | 3 (16.7) | |
| Serum creatinine at onset (mg/dL) | ||||
| Median, IQR | 0.7 | 0.8 | 0.7 | |
| Range | 0.4-2.4 | 0.3-8.4 | 0.3-8.4 | |
| Estimated glomerular filtration rate at onset (n, %) | ||||
| <60 ml/min/m2 | 1 (25.0) | 7 (50.0) | 8 (44.4) | 0.39 |
| >60 ml/min/m2 | 3 (75.0) | 7 (50.0) | 10 (55.5) | 0.37 |
| Proteinuria at onset (n, %) | ||||
| Non-nephrotic range | 2 (50.0) | 2 (14.3) | 4 (22.0) | 0.12 |
| Nephrotic range | 2 (50.0) | 12 (85.7) | 14 (78.0) | 0.14 |
| Hematuria at onset (n, %) | ||||
| Microscopic | 1 (25.0) | 3 (21.4) | 4 (22.0) | 0.55 |
| Gross | 3 (75.0) | 9 (64.3) | 12 (66.7) | 0.64 |
| Serum Albumin at onset | ||||
| Median value (g/dL) | 2.9 | 2.6 | 2.75 | |
| Hypoalbuminemia (n, %) | 2 (50.0) | 12 (85.7) | 14 (78.0) | 0.14 |
| Low Serum C3 levels (n, %) | 4 (100.0) | 12 (85.7) | 16 (89.0) | 0.41 |
Kidney biopsy findings
LM showed MesPGN in 7, MPGN in 5, diffuse endocapillary proliferative glomerulonephritis (DPGN) in 3, and CGN in 3. Glomerulosclerosis was seen in 8 (44%), Interstitial Fibrosis and Tubular Atrophy (IFTA) in 5 (27.7%) and both glomerulosclerosis and IFTA in 5 (27.7%).
Isolated C3 deposits were seen on IF in 12 of which 4 had 3+ intensity of deposits. Six patients had dominant C3 (2+/3+) along with immunoglobulins [Figure 2a and b]. EM (done in 14 patients) showed subendothelial EDD in 10, subepithelial in 7, mesangial in 7 and intramembranous in 7 [Figure 2c-e]. Majority of patients had EDD in more than one location [Table 2].
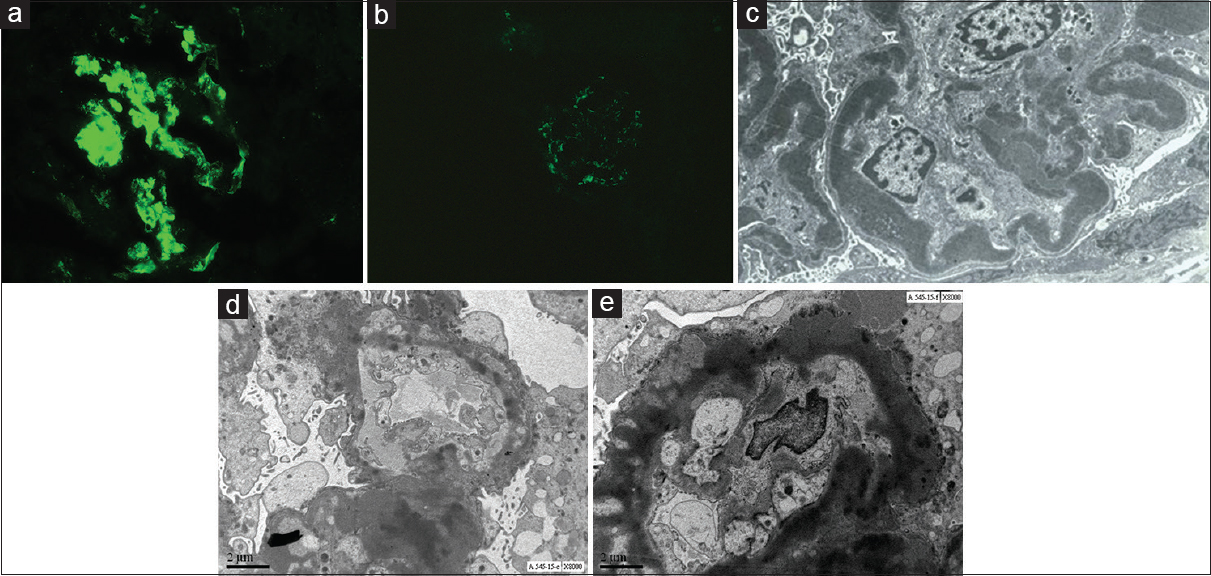
- (a) Immunoflorescence for C3 staining showing 3+ stain in the glomeruli. Original Image, Courtesy: Surgical Pathology Department, Jaslok Hospital. (b) Immunoflorescence for IgG showing 1+ staining in the glomeruli. Original Image, Courtesy: Surgical Pathology Department, Jaslok Hospital. (c) EM image showing Dense deposit disease with ribbon like intramemberanous deposits. Original Image, Courtesy: Electron Microscopy Department, Jaslok Hospital. (d) EM image of a patient with C3 glomerulonephritis, non-DDD with electron dense deposits. Original Image, Courtesy: Electron Microscopy Department, Jaslok Hospital. (e) EM image of patient with C-3 glomerulonephritis with electron dense deposits. Original Image, Courtesy: Electron Microscopy Department, Jaslok Hospital.
| Category | Dense Deposit Disease (n=4) | C3-Glomerulo nephritis (n=14) | Total (n=18) | P |
|---|---|---|---|---|
| Light Microscopic Finding | ||||
| Pattern of injury (n, %) | ||||
| Mesangial Proliferative GN | 3 (75.0) | 4 (28.6) | 7 (38.8) | 0.43 |
| Diffuse endocapillary proliferative GN | 0 (0) | 3 (21.4) | 3 (16.7) | 0.54 |
| Crescentic GN | 0 (0) | 3 (21.4) | 3 (16.7) | 0.54 |
| Membranoproliferative GN | 1 (25) | 4 (28.6) | 5 (27.7) | 0.89 |
| Exudative Glomerulonephritis (n, %) | 1 (25.0) | 11 (78.5) | 12 (66.7) | 0.42 |
| Mesangial hypercellularity (n, %) | 4 (100.0) | 11 (78.5) | 15 (83.3) | 0.32 |
| Glomerulosclerosis (n, %) | 4 (100.0) | 4 (28.6) | 8 (44.4) | 0.01 |
| Interstitial Fibrosis, Tubular Atrophy (n, %) | 1 (25.0) | 4 (28.6) | 5 (27.7) | 0.89 |
| Immunoflourescence (n, %) | ||||
| Isolated C3 | 2 (50.0) | 10 (71.4) | 12 (66.7) | 0.43 |
| C3 + Ig | 2 (50.0) | 4 (28.6) | 6 (35.7) | 0.43 |
| Electron Microscopic Finding (n, %) | ||||
| Mesangial deposits | 0 (0) | 7 (50.0) | 7 (38.9) | 0.53 |
| Subepithelial deposits/humps | 1 (25.0) | 6 (42.9) | 7 (38.9) | 0.53 |
| Subendothelial deposits | 2 (50.0) | 8 (57.1) | 10 (55.6) | 0.81 |
| Intramembranous deposits, DDD | 4 (100.0) | 3 (21.4) | 7 (38.9) | 0.006 |
Comparison of clinico-pathological profile of DDD and C3 GN
Four children (22.3%) had DDD and fourteen (77.7%) had C3GN. Mean age at presentation was 7.25 years in DDD and 7.5 years in C3 GN (P = 0.44). Male to female ratio was 3:1 in DDD and 1:1.4 in C3GN (P = 0.51).
Initial presentation was nephritic in 2/4 (50%) patients with DDD and NS in 6/14 (42.8%) in C3GN. AKI at presentation was not seen in DDD but was seen in 3/14 (21%) children with C3GN. Nephrotic range of proteinuria was seen in 2/4 (50%) patients with DDD and in 12/14 (85.7%) with C3GN. Hematuria was seen all patients with DDD with ¾ (75%) having gross hematuria. Hematuria was seen in 12/14 (85.7%) with C3GN with 9/14 (64%) showing gross hematuria. Serum C3 levels were low in all 4 (100%) DDD patients and in 12/14 (85.7%) in C3GN group (P value = 0.41). eGFR less than 60 ml/min/1.73 m2 was seen in ¼ (25%) of patients with DDD and in 7/14 (50%) in C3GN [Table 1].
The predominant histopathological pattern in DDD was MesPGN (75%) while in C3GN, MesP GN, MPGN, DPGN, and CGN were seen almost equally distributed (P = 0.43). Crescentic GN was seen in 3/14 (21%) in C3GN and in none in DDD. Glomerulosclerosis was seen in all 4 patients with DDD and in 4/14 (28.6%) patients with C3GN (P = 0.01). IFTA was seen in ¼ (25%) of children with DDD and in 4/14 (28.6%) of patients with C3GN (P = 0.89). Isolated C3 deposits were seen in 2/4 (50%) of patients with DDD and in 10/14 (71.4%) of patients with C3GN (P = 0.43) [Table 2].
Treatment
All patients received daily oral prednisolone of 2 mg/kg for 4 weeks followed by alternate day steroids at 1–2 mg/kg for prolonged periods with a variable tapering schedule. Seven patients received only long-term steroids. Five patients received long-term steroids with mycophenolate mofetil. Six patients received steroids along with other immunosuppressive drugs such as cyclophosphamide, calcineurin inhibitors with or without mycophenolate either singly or sequentially for a variable duration [Table 3].
| Treatment Received | Patient number (s) n (%) | Response | ||
|---|---|---|---|---|
| Complete response (n) | Partial response (n) | No response (n) | ||
| Steroids Alone (1st Line) | 7 (38.8%) | 2 | 1 | 4 (2 Dead, 1 ESRD, 1 LTFU) |
| Steroids + Mycophenolate mofetil | 5 (27.7%) | 3 | 2 | |
| Steroids + additional immunosuppressive (single/multiple) | 6 (33.3%) | 2 | 4 | |
| CYC | 1 | 1 (ESRD) | ||
| CNI | 2 | 2 (ESRD) | ||
| CYC followed by MMF followed by CNI | 2 | 1 | 1 (Dead) | |
| CNI followed by MMF | 1 | 1 | ||
| Total Outcome | 18 | 5 | 5 | 8 |
CR: Complete Response; PR, partial response; NR: No response; CYC: Cyclophosphamide; CNI: Calcineurin inhibitors; MMF: Mycophenolate Mofetil; ESRD: end stage renal disease; LTFU: lost to follow up
Response to therapy
Among patients on long-term steroids alone (7), CR was seen in 2, PR in 1 and no response in 4. In the patients with no response, 1 progressed to ESRD, 2 died, 1 was lost to follow up. Among patients on steroids with MMF (5), 3 achieved CR and 2 achieved PR. None progressed to ESRD or died in this subgroup. Among the group who received other immunosuppressants (6), none achieved CR; PR was seen in 2 and no response in 4. In this group 3 progressed to ESRD (2 on CNI and 1 on cyclophosphamide) and 1 died [Table 3].
Patient outcome
None of the 10 patients who achieved CR or PR progressed to ESRD or died. Of the 8 patients who showed no response; three died (2 in the first 6 months and one in the 4th year of disease), four developed ESRD and one was lost to follow up [Table 3]. All three patients who died had renal failure. Two died of flash pulmonary edema and one due to catheter associated blood stream infection.
Renal outcome
Duration of follow up ranged from 2 to 138 months with a median of 38.2 months. Kaplan Meir survival analyses showed that the 1-year patient survival was 88.5%, 5 and 10 years survival was 70.8% respectively. The renal survival without progression to ESRD was 60.2% at 1 year, 48.2% at 5 and 10 years, respectively. (HR 0.38,95% C.I.: 0.1–1.4, P = 0.1) [Figure 3] The survival curves between DDD and C3GN were not statistically different ((HR = 0.44, 95%CI = 0.09-2.23, P = 0.43).
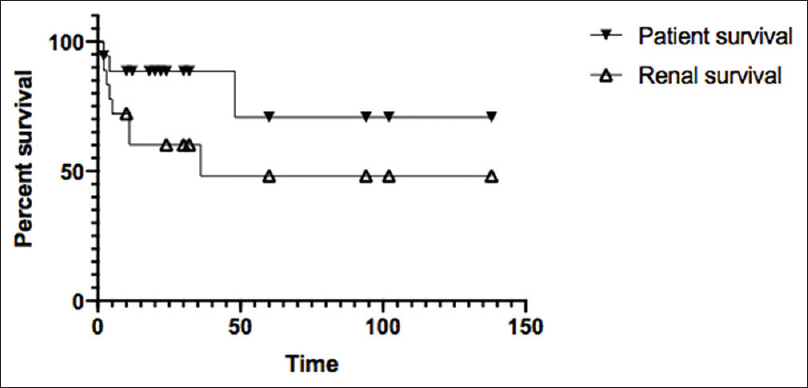
- Comparing the absolute patient survival and renal survival of patients without progression to ESRD in the C-3 Glomerulopathy cohort. The upper curve shows absolute survival of patients, with 1-year survival @ 88.5% and the 5-and 10-year survival @ 70.8%. The lower curve is to represent the renal survival without progression to ESRD, with 1-year, 5-year survival being 60.2% and 48.1%, respectively. (P value = 0.1; HR 0.38, C.I.: 0.1-1.4)
The renal survival at 5 years in children with initial presentation with hematuria was 80% as compared to those with initial presentation as nephrotic (57%), nephritic (0%) or AKI (33.3%) (P = 0.4) [Figure 4]. Using the Cox regression hazard model, the only independent variable that was identified to adversely affect the renal survival was IFTA on biopsy (P = 0.013, HR 8.1; 95%CI 1.6-42).
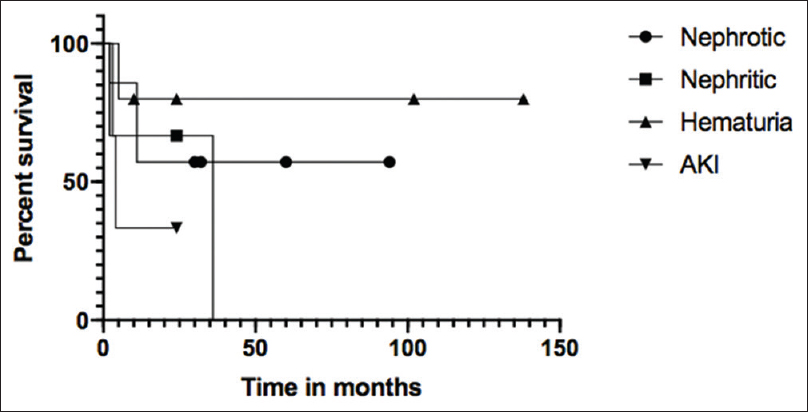
- Comparing the renal survival of C-3 Glomerulopathy patients based on their different symptoms at presentation. The curves above show no statistically significant difference (P value = 0.4) in the renal survival. The renal survival without progression to ESRD in hematuria patients was 80%, nephrotic 57%, AKI 33% and nephritic 0%
Discussion
In the last 15 years, there have been several large published case series of adults with C3GN.[7891011] Pediatric case series have been small and have not included many prepubertal children. Differences have been noted in the clinicopathological phenotype between adults and children. Long-term outcome is variable in both, with a high rate of progression to ESRD over 10 years.
Although C3GN is an uncommon entity, its prevalence appears to be greater in children than in adults. C3GN accounted for 5.3% of all renal biopsies in our study and 4.6% in another pediatric series.[12] This is higher than the prevalence of around 1% in most adult studies.[34810] We do not know if this indicates an increased prevalence of C3GN in children or is merely a reflection of more restrictive indications for biopsy in children when compared to adults.
We found that the triad of hematuria, proteinuria and low C3 was a sensitive marker for the presence of C3GN. It was seen in 88% of all our patients and in 100% of patients with DDD. The common finding of hematuria, proteinuria and low C3 has also been noted in other pediatric studies.[78] C3 was normal in two of our patients. The presence of C3GN in the absence of low serum C3 is now a known entity in certain genetic variants such as CHFR gene mutations.[813] Low C3 is less common in adults and was found in only sixty six percent of the patients in a large series.[8]
As noted in most studies, C3GN was the dominant subtype seen in 77.8% of our patients.[8111415] DDD was seen in twenty-two percent of our cohort. DDD has been reported as an ultra-rare disease that is slightly more common in children and in young adults. Hematuria was seen in all cases of DDD. Although non-nephrotic proteinuria was seen at presentation in half the cases, the proteinuria increased to nephrotic range at follow up.
MPGN is often considered as the typical light microscopic finding in C3GN. However, it is not always the commonest light microscopic change seen in all C3GN studies, especially in children.[1617] Walker et al. in a review of 80 cases of DDD (74% children) from different parts of the world concluded that DDD is not synonymous with MPGN and that MesPGN was the commonest LM finding seen in 45% of cases.[17] In the study by Nasr et al., MPGN was the commonest pathology in adults but MesPGN was seen as commonly as MPGN in children.[7] The most common morphology on LM in our study was MesPGN, seen in 75% of our children with DDD.
The prevalence of chronicity changes in our study was much higher than what is reported in other series suggesting delayed referral and late recognition of the disease.[7] In a Korean study of nine children with DDD, no patient had evidence of chronicity on histopathology and none progressed to ESRD. This may have been due to very early detection as these children were diagnosed in a school screening program.[18]
Forty percent of our children with C3GN had a steep progression to ESRD in the first year after diagnosis. Steep progression was noted by Lu et al. in DDD with 25% reaching ESRD by 2.5 years and 33% by 5 years.[9] In our patients the rapid decline was commoner in C3GN than in DDD probably related to the occurrence of crescentic GN in this group. Children with crescentic GN and AKI at onset failed to recover despite pulse steroids, cyclophosphamide with plasma exchange, and progressed to ESRD. Published studies on the results of plasma infusions for factor deficiencies and plasma exchange with immunosuppression for antibody mediated C3GN have shown benefit in some but not in all patients.[1920] Anticomplement therapy with Eculizumab, a C5a inhibitor, to prevent formation of membrane attack complexes seems an attractive option for the disease that is mediated by complement pathway dysfunction. Dramatic improvement has been noted in many, but not all patients have benefitted.[122122] The impact of Eculizumab in more chronic cases is not uniformly favorable.
Currently, there are no evidence-based recommendations for the management of this rare disease. McEnery and Adams had shown the beneficial effect of long-term alternate day prednisolone on six children with DDD who achieved complete remission.[23] Remission was also reported in 46.9% of children in Nasr's cases of DDD.[7] Two patients with DDD in our study achieved complete remission with long-term alternate day steroids. However, not all our patients had a good outcome with steroids alone.
The best response in our study was seen in five children who received long-term mycophenolate mofetil along with alternate day steroids. All five achieved remission (complete in 3 and partial in 2) with normalization of C3 levels. A Spanish study showed beneficial effects in adults treated with mycophenolate mofetil when compared to other immunosuppressive agents or no immunosuppression (80% vs 50% vs 20%).[24] Not all studies have shown good results and evidence of benefit from the use of antiproliferative drugs such as mycophenolate is conflicting.[12162425] KDIGO recommends that steroids and mycophenolate mofetil could be tried in cases with moderate severity.[16]
The overall 5-year renal survival of 52% in our study was lower than the renal survival reported in many other studies.[7814] The 5-year renal survival was better in DDD (75%) compared to those with C3GN (40%). This is at variance with most other studies which have shown that prognosis is worse in DDD when compared to C3GN. In some studies children with DDD seem to have fared better than the adults.[789] Despite the apparent better renal survival in the medium term, the long-term prognosis in our patients with DDD remains guarded as morphological changes of chronicity such as glomerulosclerosis was seen in all DDD patients. However, the only predictor of adverse outcome in our study was the presence of IFTA on renal biopsy. Adverse impact of low eGFR at presentation and IFTA on biopsy have also been reported in other studies.[11]
In conclusion, C3 glomerulopathy in children is a serious disease with significant risk of progression to ESRD. The beneficial role of early introduction of long-term steroids along with mycophenolate needs to be assessed in prospective studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int. 2014;85:450-6.
- [Google Scholar]
- Clinico-pathologic spectrum of C3 glomerulopathy-an Indian experience. Diagn Pathol. 2015;10:6-14.
- [Google Scholar]
- Incidence and profile of C3 Glomerulopathy: A single center study. Indian J Nephrol. 2015;25:8-11.
- [Google Scholar]
- Hematuria and Proteinuria, in Pediatric Kidney Disease. 2nd ed. Berlin: Springer Nature; 2016. p. :391-418.
- [Google Scholar]
- KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:1815.
- [Google Scholar]
- Dense deposit disease: Clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol. 2009;4:22-32.
- [Google Scholar]
- C3 glomerulopathy: Clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol. 2014;9:46-53.
- [Google Scholar]
- Clinical features and outcomes of 98 children and adults with dense deposit disease. Pediatr Nephrol. 2012;27:773-81.
- [Google Scholar]
- Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National registry of renal biopsies in children).Group of renal immunopathology of the Italian society of pediatric nephrology and group of renal immunopathology of the Italian society of nephrology. Nephrol Dial Transplant. 1998;13:293-7.
- [Google Scholar]
- C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int. 2018;93:977-85.
- [Google Scholar]
- C3 glomerulopathy in children: Is there still a place for anti-cellular immunosuppression? Nephrology (Carlton). 2019;24:188-94.
- [Google Scholar]
- Identification of a mutation in complement factor H-related protein 5 in patients of cypriot origin with glomerulonephritis. Lancet. 2010;376:794-801.
- [Google Scholar]
- Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454-64.
- [Google Scholar]
- C3 Glomerulonephritis associated with monoclonal gammopathy: A case series. Am J Kidney Dis. 2013;62:506-14.
- [Google Scholar]
- Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a “kidney disease: Imporving global outcomes” (KDIGO) controversies conference. Kidney Int. 2017;3:539-51.
- [Google Scholar]
- Dense deposit disease is not a membranoproliferative glomerulonephritis. Mod Pathol. 2007;20:60516.
- [Google Scholar]
- Dense deposit disease in korean children: A multicenter clinicopathologic study. J Korean Med Sci. 2012;27:1215-21.
- [Google Scholar]
- Management of Membranoproliferative glomerulonephritis type II with plasmapheresis. J Clin Apher. 2002;17:135-7.
- [Google Scholar]
- Acute renal failure in dense deposit disease: Complete recovery after combination therapy with immunosuppressant and plasma exchange. Clin Nephrol. 2011;75(Suppl 1):4-10.
- [Google Scholar]
- Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7:748-56.
- [Google Scholar]
- Patterns of clinical response to eculizumab in patients with C3 glomerulopathy. Am J Kidney Dis. 2018;20:1-9.
- [Google Scholar]
- Regression of membranoproliferative glomerulonephritis type II (dense deposit disease): Observations in six children. Am J Kidney Dis. 1988;12:138-46.
- [Google Scholar]
- Effectiveness of mycophenolate mofetil in C3 glomerulonephritis. Kidney Int. 2015;88:1153-60.
- [Google Scholar]
- Treatment options for C3 glomerulopathy. Curr Opin Nephrol Hypertens. 2013;22:231-7.
- [Google Scholar]







