Translate this page into:
Utility of Urinary Neutrophil Gelatinase Associated Lipocalin (NGAL) in Decompensated Cirrhosis
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Aims:
Renal failure occurring in the setting of cirrhosis increases mortality by more than threefold. Serum creatinine, the conventional marker for renal dysfunction has inherent limitations in identifying and categorizing renal dysfunction in patients with chronic liver disease (CLD). Neutrophil gelatinase associated lipocalin (NGAL) is a novel biomarker which gets upregulated as early as 2-6 hours following the insult to renal tubules. In this study, we aim to check the utility of uNGAL to identify the different phenotypes of renal dysfunction in patients with CLD. We also intend to assess the utility of NGAL to predict 90-day transplant-free survival in patients with CLD.
Methods:
A total number of 120 adult patients, with cirrhosis of liver were recruited. Those with pre-existing renal parenchymal disease, receiving nephrotoxic medications, spontaneous bacterial peritonitis, septic shock, proteinuria, hematuria, urinary tract infection and anuria were excluded. Urine samples for NGAL was measured at admission and at 48 hours thereafter. Patients were followed up for 90 days post admission.
Results:
Among the study population, 16 patients (13.3%) had normal kidney function, 43 (35.8%) had prerenal azotemia and 54 (45%) had Hepatorenal Syndrome (HRS - AKI) and 7 (5.8%) had acute tubular necrosis (ATN). Urinary NGAL (uNGAL) levels were considerably lower in patients with normal kidney function and prerenal azotemia. An uNGAL level of 124 ng/ml on admission could distinguish severe forms of renal injury, with a sensitivity of 86% and specificity of 84%. The non survivors had higher uNGAL levels at admission [209.6 ng/ml (118.7-376.8) vs. 123 (33.6-344.3); P = 0.013].The receiver operated curves for uNGAL and serum creatinine at admission did not show any significant difference for predicting 90 day mortality (AUC for uNGAL: 0.632 vs 0.580 for serum creatinine; difference in AUC 0.053, P value 0.17).
Conclusion:
uNGAL levels are elevated in patients with HRS-AKI and ATN. A higher uNGAL level at admission was suggestive of severe renal dysfunction. An elevated uNGAL on admission is associated with inferior survival. However, uNGAL is not superior to serum creatinine in predicting 90-day mortality.
Keywords
Cirrhosis of liver
neutrophil gelatinase associated lipocalin
renal failure
Introduction
The clinical course of hepatic cirrhosis is often complicated by a number of sequelae that occur regardless of the etiology of liver disease. Patients with cirrhosis and ascites are at high risk for development of renal impairment. Renal failure occurring in the setting of cirrhosis increases mortality by more than threefold and significantly reduces the patient survival.[12] Renal failure can result from prerenal azotemia, hepatorenal syndrome (HRS), acute tubular necrosis (ATN) and rarely due to post renal causes. Prerenal azotemia represents the functional renal component whereas acute tubular necrosis involves structural damage to the kidney. Hepatorenal Syndrome (HRS)-Acute Kidney Injury (AKI) is an extreme form of functional kidney injury, unresponsive to volume repletion. Identifying the different etiologies of renal dysfunction is imperative for optimum clinical management as well as risk stratification and organ allocation. The current diagnostic criteria for diagnosing AKI in cirrhosis is based on elevations of serum creatinine. Serum creatinine is not the ideal marker of kidney function in cirrhotic patients.[3] The creatinine kinetics is significantly altered in cirrhosis. The levels remain low, as there is reduction in the hepatic as well as muscle production of creatinine, decreased hepatic conversion of creatine to creatinine, increased volume of distribution, increased tubular secretion of creatinine, and also due to drugs altering creatinine excretion. Following an insult to renal tubules, serum creatinine takes approximately 24-48 hours to rise. In scenarios involving rapid reductions in glomerular filtration rate (GFR), serum creatinine does not reliably depict the functional status of the kidney until a steady state equilibrium is reached. Elevated serum bilirubin can cause interference with Jaffe's method for estimation of creatinine. Serum creatinine does not discriminate between different causes of AKI.
Moreover, to distinguish between different types of renal dysfunction, it might be essential to monitor the rise in creatinine over a period. Application of a creatinine-based criteria can result in significant delay in the diagnosis of renal failure.
As serum creatinine has inherent limitations in cirrhosis, there is a continuous search for newer molecules which can accurately diagnose and classifying renal dysfunction. Urinary biomarkers are molecules which are upregulated following injury to renal tubules, much ahead of the elevations in serum creatinine. There is only limited information on the utility of biomarkers in the diagnosis and prognostication of renal failure in cirrhotic patients. Neutrophil gelatinase associated lipocalin (NGAL), is a member of lipocalin family and is upregulated when there is acute injury to renal tubules. NGAL can be estimated from blood and urine as early as 2-6 hours following the insult.[4] A few small single center studies have demonstrated that NGAL levels can distinguish between prerenal, HRS and acute tubular necrosis.[567] In the current study we examined the utility of urine NGAL (uNGAL) to distinguish between different etiologies of renal dysfunction in patients with cirrhosis and its ability to predict the 90-day mortality in patients with cirrhosis and ascites.
Methods
This was a prospective observational study done in a tertiary care center from September 2014 to June 2016. All participants were more than 18 years of age, admitted to the medical wards, with cirrhosis of liver. Those with pre-existing renal parenchymal disease (a documented e GFR <60 ml/1.73 m2 using MDRD 6 equation, or urine albumin creatinine ratio >30) receiving nephrotoxic medications, proteinuria >500mg/day, presence of >50 RBC/HPF or RBC casts in urine, urinary tract infection (urine WBC >10 per high power field or positive urine culture), anuria, and septic shock were excluded. The protocol was approved by the institute ethics committee. (JIP/IEC/SC/2014/8/620). Informed consent was obtained from participants, or legally accepted relatives, in case patients were unfit to give consent.
Baseline clinical and biochemical data was collected in all patients at the time of enrollment. Urine samples for uNGAL was measured at admission and at 48 hours following admission. The samples were centrifuged at 3000 rpm for 3 minutes and the sample was stored at -80°C. The Ray Bio® Human Lipocalin-2 Enzyme-linked immunosorbent assay (ELISA) kit was used for assessing uNGAL. The lower limit of detection of uNGAL was 4 ng/ml. All ELISA measurements were done in duplicate. The intraassay coefficient of variation was 8%. All patients were called for a follow up visit at 90 days. Those who did not come for follow up, information was collected over telephone.
Case definitions
Cirrhosis was diagnosed by the presence of clinical, endoscopic or sonographic evidence of portal hypertension in a patient with either a shrunken liver (<8 cm size) or altered echo texture and presence of surface nodularity on ultrasound. Pre-renal AKI was diagnosed in patients with antecedent history of volume loss (hemorrhage, excessive diuretics, gastrointestinal loses), with reductions in serum creatinine following volume expansion with saline/albumin. Acute kidney injury (AKI) was defined as an absolute increase in serum creatinine from baseline by 0.3 mg/dL within 48 hours or a serum creatinine of >1.5 mg/dL.[8] The last available creatinine values in the preceding 6 months prior to hospitalization was taken as baseline. All AKI which satisfied the (International Ascites Club) 2007 criteria, was considered as HRS -AKI. In addition to an absolute cut off of 1.5 mg/dL, an absolute increase in serum creatinine from baseline by 0.3 mg/dL within 48 hours was also considered as HRS, if the patients satisfied all other criteria.[9] Glomerular involvement was diagnosed in cases with albuminuria >500 mg/day and of active sediments (RBC, RBC Casts) in the urine. Acute tubular necrosis was diagnosed in patients who does not fit HRS- AKI criteria or with presence of renal tubular epithelial cells or casts, muddy brown or mixed casts on urine microscopy. The type of renal dysfunction was ascertained by the treating physician in consultation with the nephrologist.
Statistical methods
All categorical variables were expressed as frequencies and proportions. All continuous variables were expressed as mean with standard deviation or median with interquartile range, based on distribution. Urinary NGAL—which did not follow a normal distribution—was log transformed and compared. Categorical variables were compared with Chi-square tests and continuous variables were compared using student's T test, ANOVA with Bonferroni correction, Mann-Whitney U test or Kruskal Wallis test based on the distribution and number of groups. A logistic regression model was used to identify the independent predictors of mortality. Receiver operated curves (ROC) were constructed to assess the ability of uNGAL to distinguish the type of renal dysfunction and predict the mortality at 3 months. A pair-wise comparison of ROC curve of serum creatinine and uNGAL was done using the method described by De Long et al., 1988. A binary logistic regression analysis was done to assess the independent predictors of mortality. All statistical analysis was carried out at 5% level of significance and P value of <0.05 was considered as significant. The data were analyzed using the statistical software SPSS version 19.0 (IBM; Armonk, NY, USA) and Medcalc (MedCalc Software Acacialaan, Belgium).
Results
A total of 170 patients were screened from September 2014 to July 2016. A total number of 120 patients who fulfilled the inclusion and exclusion criteria were recruited. The reasons for exclusion included documented chronic kidney disease (n = 15), glomerular diseases (n = 8), concomitant urinary tract infection (n = 7), nephrotoxic medications (n = 4), sepsis (n = 6) and unwilling to participate (n = 10). The baseline clinical characteristics of the subjects are given in Table 1.
| Parameter | Value |
|---|---|
| Age | 47.5 (45.2,49.9) |
| Male gender, n (%) | 102 (85) |
| Etiology of liver disease n (%) | |
| Alcohol | 84 (70) |
| HBV infection | 11 (09) |
| HCV infection | 03 (05.8) |
| Cryptogenic | 14 (11.7) |
| Autoimmune | 01 ( 0.8) |
| Others | 03 (2.5) |
| Child-Turcot-Pugh class, n (%) | |
| Class B | 70 (58) |
| Class C | 50 (42%) |
| Complications, n (%) | |
| Hematemesis/Malena | 64 (53.3) |
| Hepatic encephalopathy | 47 (39.1) |
| Ascites | 93 (77.5) |
Among the study population, 16 patients (13.3%) had normal kidney function, 43 (35.8%) had prerenal azotemia and 54 (45%) had HRS -AKI and 7 (5.8%) had ATN. Ours being a referral hospital, the proportion of cases of cirrhosis with complications like HRS is higher than that in the general population. Among the 61 patients who satisfied AKI definition, 14 were diagnosed by an increase of creatinine >0.3 mg/dL over baseline and 47 patients had serum creatinine >1.5 mg/dL on admission. The uNGAL levels were considerably lower in patients with normal kidney function and prerenal azotemia [Table 2]. The trends in serum creatinine and uNGAL in first 48 hours are given in Figure 1. The AUC for uNGAL at admission and 48 hours to distinguish between normal kidney function/prerenal azotemia from severe forms of renal involvement (HRS -AKI & ATN) was 0.890 and 0.880 (P < 0.001) respectively, with 95% Confidence Interval of 0.83-0.95 [Figure 2]. An uNGAL cutoff of 124 ng/ml on admission could distinguish between normal kidney function and prerenal azotemia from severe forms of renal involvement with a sensitivity and specificity of 86% and 84% respectively. Positive predictive value was 76.1% and negative predictive value was 84.9%. Positive likelihood of AKI at urinary NGAL of 124ng/ml was 5.37 while negative likelihood ratio was 0.16. Diagnostic accuracy was 0.8.
| Parameter | Normal renal function, n=16 (Group 1) | Prerenal Azotemia n=43 (Group 2) | HRS-AKI n=54 (Group 3) | Acute tubular necrosis, n=7 (Group 4) | P |
|---|---|---|---|---|---|
| Urea (mg/dL) Median, IQR | 17 (15-25) | 28 (17-40) | 60 (36.25-88.5) | 79 (39-111) | 0.000 |
| Creatinine (mg/dL) Mean, SD | 0.82, 0.17 | 1.06, 0.25 | 2.271.29 | 2.24, 0.93 | 0.000 |
| uNGAL at admission (ng/mL) Median, IQR | 43.2 (17.2-68.6) | 76 (38-142.3) | 321.3 (182.6-486.5) | 356.9 (143-604) | 0.000 |
| uNGAL 48 hrs (ng/mL) Median, IQR | 35.4 (16.7-74.9) | 76 (49-132.9) | 312.6 (190.7-436.6) | 406 (156-628.9) | 0.038 |
| Change in uNGAL in 48 h (ng/mL) Median, IQR | 2.7 (-4.6, 12.8) | -7.3 (-32.8, -18.2) | 2.7 (-4.6, 12.8) | 19.6 (-14.8, 74.9) | 0.336 |
| MELD score Median, IQR | 11 (09-30.75) | 18 (12-21) | 20 (16-32.75) | 19 (17-38) | 0.005 |
The uNGAL VALUES were log transformed values were compared using ANOVA. *uNGAL – HRS-AKI vs ATN, P=1.00; HRS-AKI vs prerenal azotemia, P=<0.01, HRS-AKI vs Normal, P=<0.01

- uNGAL trends in first 48 hours among subgroups
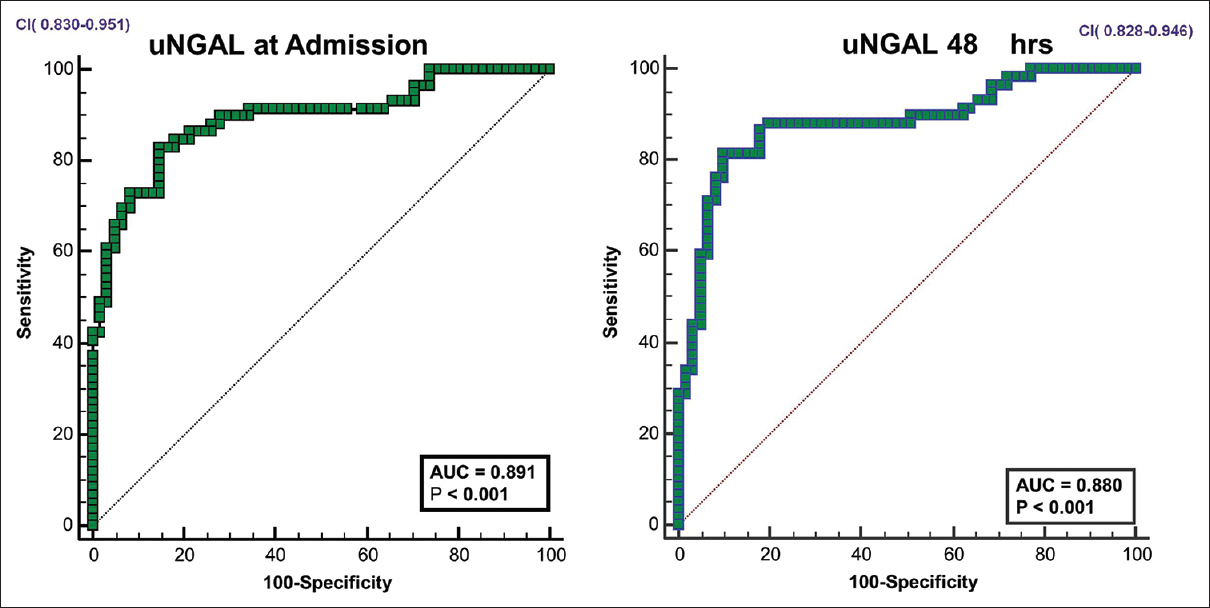
- ROC curves for u NGAL in predicting HRS-AKI/ATN
Follow-up data was available for 107 patients. Mortality in the participants with prerenal azotemia was 18 out of 35 (51.4%), HRS-AKI was 31 out of 54 (57.4%), ATN was 5 out of 7 (71.4%) and those with normal renal function was 6 out of 11 (54.5%). Data was missing for 8 patients in the prerenal group and 5 in those with normal renal function. The characteristics of survivors and non-survivors are given in Table 3. The parameters like hemoglobin, platelet count, bilirubin and liver enzyme levels were not different between survivors and non-survivors. Receiver operated curves (ROC) were plotted for serum creatinine and uNGAL on day 1 to predict the 90-day mortality. For serum creatinine, AUC was 0.580, but it did not reach statistical significance. The AUC for uNGAL on admission was 0.632, P = 0.019 [Figure 3]. A pair wise comparison of ROC curve did not show better performance for uNGAL (difference in AUC 0.0527, P value 0.17). The mortality was not different between different categories (P = 0.69).
| Parameter | Entire cohort (n=120) | Survivors (n=47) | Non-survivors (n=60) | P |
|---|---|---|---|---|
| Age (Mean, SD) | 47.5, 12.8 | 49.6, 12.33 | 46.9, 13.1 | 0.292 |
| Male gender, n (%) | 102 (85) | 43 (91.5) | 51 (85) | 0.207 |
| Hemoglobin (Mean, SD) | 9.4, 2.3 | 9.73, 1.8 | 9.05, 2.3 | 0.108 |
| Total count, cells/ml (Median, IQR) | 9050 (5890, 12000) | 9050 (5890, 12000) | 11100 (6685, 13550) | 0.173 |
| Platelet count, cells×103/ml (Median, IQR) | 1.1 (0.66-1.97) | 1.0 (0.57-1.93) | 1.09 (73.5-1.99) | 0.751 |
| AST (Median, IQR) | 65 (38-112.5) | 56 (37-92) | 70 (40-127) | 0.184 |
| ALT (Median, IQR) | 40 (28-57.5) | 36 (27-48) | 13.5 (29-76.5) | 0.148 |
| ALP (Median, IQR) | 295 (199-429) | 254 (188-467) | 307 (214-447) | 0.418 |
| GI bleed n (%) | 64 (53.3) | 21 (44.6) | 35 (58.3) | 0.161 |
| Hepatic encephalopathy, n (%) | 47 (39.1) | 12 (11.2) | 28 (26.1) | 0.025 |
| Bilirubin (Median, IQR) | 2.3 (0.92-7.17) | 2 (0.8-4.3) | 2.7 (1.0-10.15) | 0.135 |
| PT (seconds) (Median, IQR) | 18 (15-23) | 16 (14-21) | 18.5 (15.2-25.9) | 0.045 |
| Albumin (g/dL) (Mean, SD) | 2.63, 0.56 | 2.66, 0.6 | 2.52, 0.52 | 0.200 |
| MELD Score (Median, IQR) | 19 (13.25-25.75) | 17 (12-21) | 21 (16-32.7) | 0.002 |
| Urea (mg/dL) (Median, IQR) | 37 (18.25-70) | 33 (22-70) | 39.5 (17.25-78.7) | 0.410 |
| Creatinine (mg/dL) (Mean, SD) | 1.64, 1.1 | 1.45, 0.70 | 1.95, 1.36 | 0.015 |
| uNGAL at admission (ng/mL)* (Median, IQR) | 143 (60.9-353.9) | 123 (33.6-344.3) | 209.6 (118.7-376.8) | 0.013 |
| uNGAL 48 h after admission (ng/mL)* (Median, IQR) | 139 (57.9-342.3) | 128.1 (43-283.1) | 231.8 (106.3-400.56) | 0.006 |
| HRS-AKI and ATN, n (%) | 61 (50.8) | 25 (53.2) | 36 ( 60) | 0.498 |
13 patients were lost to follow up. *The log transformed values were compared using Student’s t-test
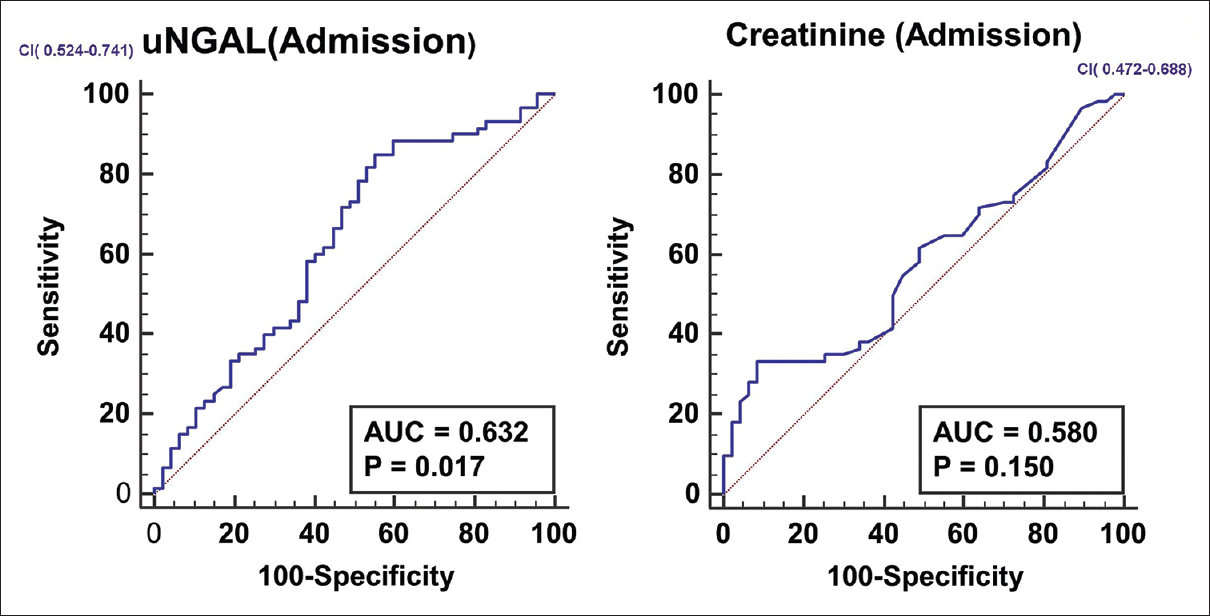
- ROC curves for serum creatinine and u NGAL in predicting 90 day mortality
A binary logistic regression analysis was done to assess the independent predictors of mortality. All variables with P value less than 0.100 were incorporated into the logistic regression model- PT, creatinine, MELD score, hepatic encephalopathy and uNGAL. [Table 4] PT, creatinine, MELD score were analysed as continuous variables where as uNGAL and hepatic encephalopathy were categorical variables. A cut-off value of uNGAL 124ng/ml was taken based on ROC curves. Since creatinine and MELD Score are not correlated terms, their inclusion in the logistic regression model did not lead to collinearity.
| Parameter | Exp(B) | 95% confidence interval | P |
|---|---|---|---|
| uNGAL >124 ng/mL | 3.238 | 1.103-9.508 | 0.033 |
| PT (seconds) | 1.057 | 0.989-1.130 | 0.102 |
| Creatinine (mg/dL) | 1.029 | 0.603-1.756 | 0.917 |
| MELD Score | 1.038 | 0.977-1.102 | 0.225 |
| Hepatic encephalopathy | 0.600 | 0.227-1.586 | 0.303 |
*The model was adjusted for age
Discussion
The current liver allocation scoring systems also rely on serum creatinine as the sole marker of renal dysfunction.[10] Depending on serum creatinine as the sole marker of kidney function can delay management due to the altered creatinine kinetics associated with liver failure. A serum creatinine value which is considered as normal in general population need not represent a state of normal renal function in patients with cirrhosis. It has been reported that the mortality in cirrhosis tends to be three-fold higher at a serum creatinine of 1 mg/dL, a value which is well within the normal limits.[11] Urine output also tends to be an unreliable marker of renal dysfunction in cirrhosis. There is an unmet need of a reliable marker which could predict renal dysfunction sufficiently early in the course of cirrhosis.
Neutrophil gelatinase associated lipocalin (NGAL), a 25 k Da glycoprotein belonging to the lipocalin family is one of the best studied urinary biomarkers in cirrhosis. Most of the earlier studies have focused on the utility of NGAL to differentiate between the different etiologies of renal dysfunction in cirrhosis. Verna et al. was the first to report that uNGAL can be used to distinguish between prerenal AKI, HRS and intrinsic AKI. They reported median u NGAL values of 20, 20, 105 and 325 ng/ml in patients with normal kidney function, pre-renal azotemia, HRS AKI and intrinsic ATN respectively. They also reported that u NGAL (>110 ng/ml) had better sensitivity in predicting in hospital mortality compared to serum creatinine and MELD score.[6] Spanish investigators reported lower values of uNGAL in prerenal azotemia, intermediate values in HRS and higher values in ATN.[1213] They found NGAL to be good predictor of mortality at 3 months.[13] Belcher et al. reported NGAL values of 115 ng/ml, which was lower than that in ATN (565 ng/ml).[14] Quasem et al. reported that uNGAL can distinguish between different types of renal failure, but the mean uNGAL values were higher. They reported u NGAL value of 161 ng/ml in pre-renal azotemia, 380 ng/ml in HRS and 580 ng/ml in ATN.[15] On comparison with the previous studies, we found that, in this study, mean u NGAL was 43.2 in normal kidney function, 76 in prerenal azotemia, 321 in HRS-AKI and 357 in ATN.
In the current study, we found that u NGAL can distinguish between normal kidney function/prerenal azotemia and HRS-AKI/ATN. However, the values were not significantly different between HRS -AKI and ATN. One potential reason for this finding might be due to the selection criteria employed for the current study. Pre-existing parenchymal renal disease was an exclusion criterion, thus eliminating patients with HRS -CKD (type 2 HRS). It is reported that in type 1 HRS with acute precipitating events like infections, the uNGAL levels tends to be 2 -10-fold higher than type 2 HRS.[1316] Mean uNGAL values as high as 725 ng/ml has been reported in patients with HRS –AKI.[16] This may account for the uNGAL values being similar in HRS-AKI and ATN. uNGAL is reported to be a better predictor of short-term mortality compared to serum creatinine and MELD score.[131617]
In our study, uNGAL was found to be an independent predictor of mortality, similar to earlier studies. The 3-month mortality rates in the current study is higher when compared to the existing literature. This was probably because we included cirrhosis with higher CTP class (B and C) as study participants.
To clinically classify the type of renal dysfunction based on available clinical criteria, we had to wait for 48 hours to assess the trends in serum creatinine. We found an uNGAL cutoff of 124 ng/ml at admission would cut short this time interval needed for the subtyping of renal failure. There are inherent dilemmas in putting AKI in cirrhosis to water tight compartments. Even though a formal diagnostic criterion has been laid down for the diagnosis of HRS-AKI, at times, the distinction between HRS- AKI, prerenal azotemia and ATN can be challenging. The current concepts of AKI in cirrhosis is a simplistic view, with emphasis on renal perfusion as the sole factor for the genesis of renal dysfunction.[1819] Both HRS-AKI, prerenal azotemia and ATN can have common triggers like spontaneous bacterial peritonitis and infections. Endothelial injury from infectious triggers may play an equally important role as reduced renal blood flow.[181920] The existing classification schema does not take in to account factors like intraabdominal hypertension and normotensive ischemic ATN and biliary casts which may contribute to renal dysfunction in cirrhosis.[182021] Fractional excretion of sodium is often unreliable due to concomitant diuretic usage. Urine microscopy is often not standardized and interpretation of sediment can be complicated by the bilirubin staining.[22] It is reported that, in up to 42% patients with a presumed diagnosis of HRS, kidney function does not completely recover following orthotopic liver transplantation.[2324] These patients might be potentially having ATN or even CKD which has been misclassified as HRS. Moreover, the uNGAL kinetics does not parallel the creatinine. uNGAL tend to peak around 24 hours following tubular injury, whereas creatinine takes up to 48 hours or longer to peak.[25] These factors might act as a limitation to find a cut off value of u NGAL to distinguish between HRS and ATN, using a serum creatinine-based criterion as the reference.
Interestingly, in the current study, we found that mortality was not different between the different groups. About half of the patients with normal kidney function expired by the end of three months. These patients were considered to have normal kidney function, as they had no precipitating events and the serum creatinine had remained static. Milder degrees of renal dysfunction and early CKD can be often overlooked in cirrhosis, due to the inherent limitations of creatinine as a filtration marker.[1118] This emphasizes the need to diagnose AKI early using biomarkers like NGAL in lieu of creatinine.
The current study has a few limitations. The u NGAL was measured only at two points – on admission and 48 hours. Patients with sepsis and hypotension were excluded. We do not have data on uNGAL kinetics throughout the hospital stay. A direct method of measuring the GFR would have given better information on kidney function.
Conclusion
Urinary NGAL levels tend to be higher in patients with HRS-AKI and ATN. However, it is not superior to serum creatinine in predicting 3-month mortality rates in patients with decompensated cirrhosis.
Compliance with ethical requirements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Funding agency and Grant no: JIPMER, JIP/RES/Intra-MD-MS/ first/04/2014/no: 135.
Conflicts of interest
There are no conflicts of interest.
References
- Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol. 2006;44:217-31.
- [Google Scholar]
- Neutrophil gelatinase-associated lipocalin (NGAL): A new marker of kidney disease. Scand J Clin Lab Invest Suppl. 2008;241:89-94.
- [Google Scholar]
- Biomarkers for acute kidney injury in decompensated cirrhosis: A prospective study. Nephrology (Carlton). 2019;24:170-80.
- [Google Scholar]
- Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci. 2012;57:2362-70.
- [Google Scholar]
- Diagnostic approach to renal failure in cirrhosis. Clin Liver Dis (Hoboken) 2013:128-131.
- [Google Scholar]
- Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310-8.
- [Google Scholar]
- Model for end-stage liver disease score and MELD exceptions: 15 years later. HepatolInt. 2015;9:346-54.
- [Google Scholar]
- “Normal” creatinine levels predict persistent kidney injury and waitlist mortality in outpatients with cirrhosis. Hepatology. 2018;68:1953-60.
- [Google Scholar]
- Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;572:267-73.
- [Google Scholar]
- Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J Hepatol. 2014;61:35-42.
- [Google Scholar]
- Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology (Baltimore, Md). 2014;60:622-32.
- [Google Scholar]
- Urinary biomarkers of acute kidney injury in patients with liver cirrhosis. ISRN Nephrology. 2014;2014:376-95.
- [Google Scholar]
- Neutrophil gelatinase-associated lipocalin in prediction of mortality in patients with hepatorenal syndrome: A prospective observational study. Liver Int. 2014;34:49-57.
- [Google Scholar]
- Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol. 2016;65:57-65.
- [Google Scholar]
- Acute kidney injury in patients with cirrhosis: Perils and promise. Clin GastroenterolHepatol. 2013;11:1550-8.
- [Google Scholar]
- International club of ascites.Current limits and future challenges in the management of renal dysfunction in patients with cirrhosis: Report from the International club of ascites. Liver Int. 2013;33:16-23.
- [Google Scholar]
- Renal dysfunction in cirrhosis is not just avasomotor nephropathy. Kidney Int. 2015;87:509-15.
- [Google Scholar]
- Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192-7.
- [Google Scholar]
- Diagnostic utility of urine microscopy in the differential diagnosis of acute kidney injury. Clin Am J Soc Nephrol. 2008;3:1615-9.
- [Google Scholar]
- Impact of the etiology of acute kidney injury on outcomes following liver transplantation: Acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539-48.
- [Google Scholar]
- The course of type 1 hepato-renal syndrome post liver transplantation. Nephrol Dial Transplant. 2006;21:478-82.
- [Google Scholar]
- Urinary neutrophil gelatinase-associated lipocalin time course during cardiac surgery. Ann Card Anaesth. 2015;18:39-44.
- [Google Scholar]







