Translate this page into:
Plasmablastic Lymphoma Involving Kidney in an HIV Positive Patient: A Case Report with Review of the Literature
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Plasmablastic lymphoma (PBL) is an aggressive lymphoma commonly associated with HIV infection. It most commonly presents in the oral cavity and rarely involves the kidney. Herein, we report a case of HIV positive male with renal involvement of PBL. The patient presented with unilateral severe hydronephrosis with unaltered renal functions. Despite aggressive management, there was an early relapse and the patient died within 2 years of the diagnosis. Despite the recent advances in the therapy of HIV-associated aggressive lymphomas, patients with PBL have a poor prognosis. Multimodal treatment with chemotherapy, newer targeted and biological agents, along with hematopoietic stem cell transplantation is essential for the treatment of PBL.
Keywords
HIV- positive
kidney
plasmablastic lymphoma
Introduction
Plasmablastic lymphoma (PBL) is a distinct clinicopathological entity that was initially described in 1997.[1] It is now considered a distinct subtype of diffuse large B-cell lymphoma (DLBCL) seen more commonly in patients with HIV infection.[2] A common presentation is lesions in the oral cavity. Microscopically, it is characterized by cytomorphologic features such as large immunoblasts or large plasma cells, that express CD79a, IRF-4/MUM-1, BLIMP-1, CD38, and CD138 but negative for B-cell markers CD19, CD20, and PAX-5.[3] The prognosis is relatively poor with a median overall survival (OS) of 15 months and a 3-year OS rate of 25%.[4] Autologous or allogeneic hematopoietic stem cell transplant has been considered in advanced disease with ambiguous prognostic benefits.
Case Report
A 53-year-old HIV positive male presented with a history of pain in the left lower back radiating to leg for 2 months duration. He was a known diabetic, hypertensive, and had taken treatment for pulmonary tuberculosis in the past. He was on antiretroviral therapy (ART) along with prophylactic treatment for pneumocystis carinii.
On examination, he was moderately built with Eastern Co-operative Oncology Group (ECOG) performance status 1. His abdomen examination revealed a firm, tender mass occupying the left hypochondrium along with diffuse abdominal distension. His blood parameters and biochemical reports including renal function tests were normal. Sputum was negative for tuberculosis and HIV viral load was lower than the detectable limits (Genexpert -PCR) 1 copy/mL = 1.71 IU/mL. His CD4 count was 207 cells/mcL. Positron emission tomography-computed tomography (PET-CT) scan [Figure 1] showed a 10.0 × 11.6 × 20.1 cm metabolically active soft tissue density mass lesion in the left perinephric space, significantly in the lower pole and infiltrating the renal fascia, extending to the lower hilum and encasing the proximal and mid ureter with resultant luminal narrowing with resultant left hydronephrosis, suggestive of lymphoma. Multiple small metabolically active retroperitoneal, pelvic, mesenteric, mediastinal, hilar, and cervical lymph nodes were noted. Loculated right pleural collection with trace ascites was also seen.

- PET CT scan showing left renal involvement
He underwent a CT-guided core biopsy of the perinephric mass, which revealed diffuse malignancy, characterized by a relatively monotonous population of dyscohesive lymphoplasmacytoid cells suggestive of non-Hodgkin's lymphoma [Figure 2]. Immunohistochemistry of the tumor showed positive CD138, MUM-1, Ki-67 was 90%. EMA, CD20, CD30, CK, and EBER were negative. The IHC supported the diagnosis of PBL. Bone marrow biopsy revealed hypercellular marrow with features of erythroid hyperplasia and megaloblastic maturation with no definite evidence of lymphomatous involvement.
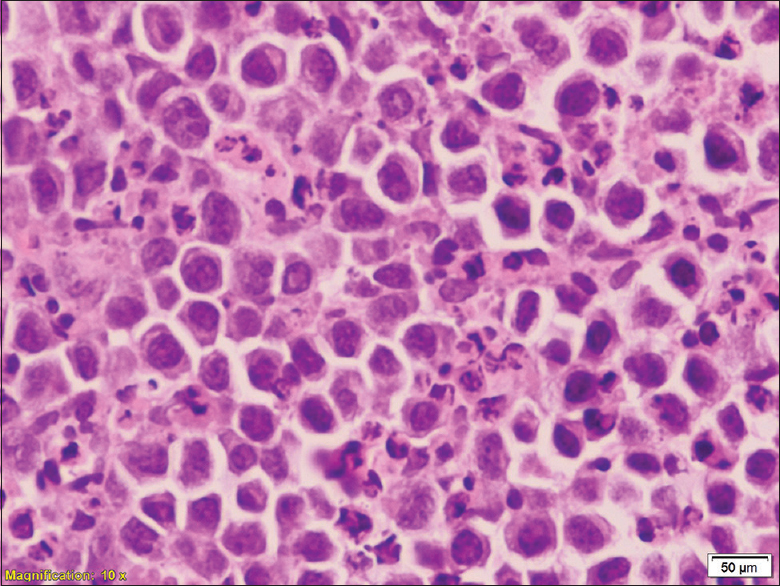
- HP showing plasmablastic lymphoma (Confirmed by IHC magnification 10×)
Hence, the diagnosis of PBL stage IVB with a high international prognostic index (IPI) score was established. He was planned for CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone) based systemic chemotherapy with intrathecal chemotherapy followed by autologous stem cell transplant. He received three cycles of chemotherapy and was reassessed with a PET-CT scan, which showed near-complete resolution of left perinephric and retroperitoneal mass lesions with significant regression of other lymph nodes. He completed six cycles of chemotherapy and on evaluation with PET-CT scan, had stable disease. We planned for autologous stem cell transplant for consolidation, but it was deferred due to financial constraints.
After 2 months, PET-CT scan showed a new lesion in the pancreas with SUVmax – 14.7 and left posterior inferior pararenal space/psoas muscle with SUVmax – 12.7, suggestive of relapse. Given the early relapse and aggressive nature of the disease, he was planned for second-line chemotherapy with ICE (ifosfamide, carboplatin, and etoposide) regimen followed by autologous stem cell transplant. His disease progressed further, and he succumbed to death within 2 years of treatment.
Discussion
PBL is AIDS-defining cancer, classified by WHO as a distinct entity of aggressive DLBCL.[5] Pathogenesis is related to immunodeficiency, chronic immune stimulation as well as due to oncogenic virus-like Ebstein Bar Virus (EBV). This pattern of chronic humoral immune-stimulation and cellular immunodeficiency was seen in our case; however, EBV was not detected. In HIV positive patients, the oral cavity is the frequently involved site (58%), bone marrow involvement (30%) has been reported in both HIV-positive and HIV-negative patients.[67] Other less common extraoral sites are central nervous system (CNS), paranasal sinus, mediastinum, lungs, liver, and testes. PBL is frequently associated with low CD 4 count and high viral load, this finding was in contrast to the findings to our case, where the viral load was negligible and CD4 count was moderately low. A similar case of PBL in the immunocompetent patient which originated from psoas muscle was reported by Thakral et al.[8]
In PBL, the use of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) are considered substandard therapy, and recent guidelines recommend more intensive regimens such as etoposide, vincristine, and doxorubicin with bolus cyclophosphamide and prednisone (EPOCH).[9] It is known that patients with PBL with the chemotherapy-responsive disease, might benefit from autologous SCT in first remission, however, the use of allogeneic SCT in HIV-positive PBL shows limited efficacy. We had managed our case aggressively with CHOEP chemotherapy protocol and had planned for autologous stem cell transplant, despite aggressive treatment, our case relapsed early and died within 2 years of diagnosis.
Novel agents for the treatment of PBL include bortezomib, immunomodulators like lenalidomide and adoptive cellular therapy which are indicated for a broad range of hematological malignancies.[10] About 30% of PBL cases express the activation marker CD30.[11] Holderness et al.[12] reported a case of a relapsed PBL expressing CD30, who responded to brentuximab vedotin.
Conclusion
PBL carries a very poor prognosis with resistance to conventional treatment. Multidisciplinary modality is essential for treatment. Novel treatment strategies include target therapy, adoptive cellular therapy, immune checkpoint inhibitors which are promising options in the future. The involvement of the kidney is a rare finding which might cause a dilemma in diagnosis as well as delay in the treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Plasmablastic lymphomas of the oral cavity: A new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413-20.
- [Google Scholar]
- Plasmablastic lymphoma. In: Swerdlow S, Campo E, Harris NL, eds. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. p. :256-7.
- [Google Scholar]
- Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol. 2005;18:806-15.
- [Google Scholar]
- HIV-associated plasmablastic lymphoma: Lessons learned from 112 published cases. Am J Hematol. 2008;83:804-9.
- [Google Scholar]
- WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th ed). France: IARC Press; 2017.
- Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk Lymphoma. 2010;51:2047-53.
- [Google Scholar]
- Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol. 2004;28:736-47.
- [Google Scholar]
- AIDS-Related B-Cell Lymphomas. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf
- Chimeric antigen receptor modified T cell therapy for B cell malignancies. Int J Hematol. 2014;99:132-40.
- [Google Scholar]
- Plasmablastic lymphoma: A clinicopathologic correlation. Ann Diagn Pathol. 2006;10:8-12.
- [Google Scholar]
- Brentuximab vedotin demonstrates activity in a patient with plasmablastic lymphoma arising from a background of chronic lymphocytic leukemia. J Clin Oncol. 2013;31:e197-9.
- [Google Scholar]







