Translate this page into:
Effects of Rosmarinic Acid on Methotrexate-induced Nephrotoxicity and Hepatotoxicity in Wistar Rats
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Methotrexate (MTX), used in the treatment of cancerous patients, causes toxicity in the different organs of the body. This study of rosmarinic acid (RA) is as an antioxidant on nephrotoxicity and hepatotoxicity induced by MTX.
Methods:
Rats (n = 32) were divided into four groups: sham; MTX; 100 mg\kg RA + MTX; 200 mg/kg RA + MTX. The amount of MTX was 20 mg/kg. 24 hours after injection of the last dose of MTX, the blood samples and kidneys and liver of rats were studied. The aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), urea, serum creatinine were assessed. Tissue antioxidant enzymes and malondialdehyde (MDA) levels were measured. The liver and kidney tissues were histopathologically examined.
Results:
MTX significantly increased the urea, creatinine, ALT, AST, ALP levels, and renal MDA and significantly decreased renal catalase (CAT), hepatic glutathione (GSH), and hepatic CAT activity. MTX induced necrosis, leukocyte infiltration, eosinophilic casts, glomerular damage in kidney tissue and necrosis, degeneration and cellular vacuolization in liver tissues. RA at 100 mg/kg caused a significant decrease in ALT and AST and at two doses significantly decreased urea, renal MDA, and liver MDA. RA at 200 mg/kg significantly increased the renal CAT and liver GSH. RA in two doses significantly decreased necrosis and Leukocyte infiltration. RA caused a significant decrease in degeneration and cellular vacuolization in liver tissues.
Conclusions:
RA with its antioxidant and anti-inflammatory characteristics decreased the MTX induced nephrotoxicity and hepatotoxicity.
Keywords
Hepatotoxicity
methotrexate
nephrotoxicity
rat
rosmarinic acid
Introduction
Methotrexate (MTX) is a drug widely used in the treatment of inflammatory diseases such as psoriasis, rheumatoid arthritis, treatment of leukemia, and other cancers. This drug not only destroys cancerous cells but also causes injury in the normal tissues of the body.[123] High levels of MTX causes injuries in the vital organs such as liver and kidney with prevalence of hepatic fibrosis at 50% and hepatic cirrhosis at 26%. As MTX is excreted by renal tubules, the sediment of MTX and its metabolites also causes renal function disorders.[45] MTX produces reactive oxygen species (ROS) and reduces the efficiency of antioxidant enzymes, induces apoptosis, and destroys both cancerous and healthy cells by producing ROS and decreases the efficiency of antioxidant enzymes.[3456] Rosmarinic Acid (RA), both as strong antioxidant and phenolic compounds can be found in various plants such as Rosmarinus officinalis, Perilla frutescens (perilla), Origanum, Vulgare, Thymus vulgaris, Menthe spicata, and Ocimum basilicum.[7] Like most antioxidants, RA leads to a reduction of oxidative stress in the pathological conditions due to its antioxidant and anti-inflammatory properties.[8] Thus, the purpose of the current study was to explore the protective impact of RA on the reduction of oxidative stress and reinforcement of the antioxidant system in methotrexate-induced hepatotoxicity and nephrotoxicity.
Methods
Drugs and chemicals
The methotrexate (Sigma-Aldrich, France) and rosmarinic acid (536954/USA) were bought to perform this study.
Animals
All animal experiments were carried out based on internationally accepted guidelines for the use of animals in research and were approved by the ethics committee of Dezful University of Medical Sciences, Iran (Ethical number: IR.DUMS.REC.1396.10. Approved on October 22, 2016). Wistar rats (n = 32; weighting 200–220 g) were kept in a similar condition of a temperature of 22 ± 2 and humidity of 50 ± 10% with 12 hours light/dark cycle during this procedure in the animal lab of Dezful University of Medical Sciences.
Experimental design
Rats (n = 32) were randomly divided into four groups (n = 8 for each group).
Group 1 (sham): daily they received 0.5 ml saline intraperitoneally for 3 days.
Group 2: received 20 mg/kg MTX daily intraperitoneally for 3 days.
Group 3: received 20 mg/kg MTX + 100 mg/kg rosmarinic acid.
Group 4: received 20 mg/kg MTX + 200 mg/kg rosmarinic acid.
Rosmarinic acid daily was injected by gavage as pretreatment in each of its two doses for 12 days.[910] Methotrexate was injected intraperitoneally on the tenth, eleventh, and twelfth days along with rosmarinic acid. Twenty-four hours after injection of the last dose of MTX, the blood samples of anesthetized rats were taken then their livers and kidneys were separated to assess histological and biochemical parameters.
Renal and Liver functional tests
The blood samples of animals were centrifugated with 3000 rpm for 10 minutes. After separation of their plasma, alkaline phosphatase (ALP), aminotransferase (AST), aspartate, alanine aminotransferase (ALT), urea, serum creatinine of samples were evaluated with Pars Azmon commercial kits and autoanalyzer.
Oxidative stress markers
The left kidney and liver tissues were removed, homogenized, and ten-fold of phosphate-buffered saline (PBS) was added to them. The ultimate solution was centrifugated at 4°C with 12500 rpm for 15 minutes. The supernatant was used to assess glutathione (GSH) and glutathione peroxidase (GPX), catalase (CAT), and malondialdehyde (MDA). MDA levels of the kidney and liver were measured by the Buege method,[11] and their absorbance was read at 532 nm. Glutathione levels of liver and kidney were evaluated by the Ellman method. Then, their absorbance was read at 412 nm with a spectrophotometer instrument.[12] The GPX level of kidney and liver tissues was assessed by the Fielding method and the absorption of GPX was read at 470 nm.[13] The amount of renal and hepatic catalase was evaluated by the Aebi method and its absorbance was measured by spectrophotometer.[14]
Histopathological assessments
Renal and hepatic tissues were kept in 10% neutral formalin at least for 48 hours. After assessment with ethanol solutions and xylene, they were embedded in paraffin. The block was cut into 5-μm sections and stained with eosin and hematoxylin. Then, these were examined by a light microscope under 400 × magnification. Renal injuries were measured by such parameters as leukocyte infiltration, tubular necrosis, eosinophilic casts, and glomerular damage. The Caramelo method was used to evaluate leukocyte infiltration, tubular necrosis, and eosinophilic casts as follows:[15]
No damage = 0,
Mild = 1; unicellular, patchy isolated,
Moderate = 2; damage less than 25%,
Severe = 3; damage between 25 and 50%,
Very severe = 4; more than 50% damage
Glomerular damage was assessed based on the Cakir method. Glomerular alternations such as narrowness and destruction of Bowman's space and its clasp into Bowman's capsule were assessed by this method and were semi-quantitatively scored as follows:
0: none of these mentioned cases was observed, no damage,
1: less than 25% of gamers were affected,
2: between 25 and 50% of Gamers were affected,
3: more than 50% of gamers were affected[16]
In this study, liver tissue injuries were semi-quantitatively scored by assessment of parameters such as necrosis, degeneration, cellular vacuolization.
0: no damage 1: mild damage 2: moderate damage 3: severe damage.[17]
Statistical analysis
Data were analyzed using one-way ANOVA. The Tukey test was used to compare groups two by two. Statistical significance was accepted at P < 0.05 and values expressed as mean ± SEM. The statistical analysis was run by SPSS 23.0 and the Graph Pad Prism 6 used to illustrate the article graphs.
Result
Effects of RA on renal function
Urea and serum creatinine index in different groups is indicated in Figure 1. The urea and creatinine levels in the MTX group had significantly increased compared with the sham group. Compared with the MTX group, pretreatment with RA in its two doses decreased the urea level and this reduction in 100 mg/kg group was significant (p < 0.05). RA did not decrease the serum creatinine in both receiving groups.

- Effects of Rosmarinic acid on kidney function tests in methotrexate-induced hepatorenaltoxicity. Data were expressed mean ± SEM (n = 8). Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. * Significant difference compared to MTX group (P < 0.05), # significant difference compared to Sham group (P < 0.05)
Effects of RA on liver function
In this study, ALP, AST, and ALT were evaluated as liver function markers. The serum level of these tests was reported by graphs in Figure 2. ALT, AST, and ALP levels in the MTX group significantly increased (p < 0.05). Pretreatment with RA in 100 mg/kg could significantly decrease the AST, ALT levels (p < 0.05).
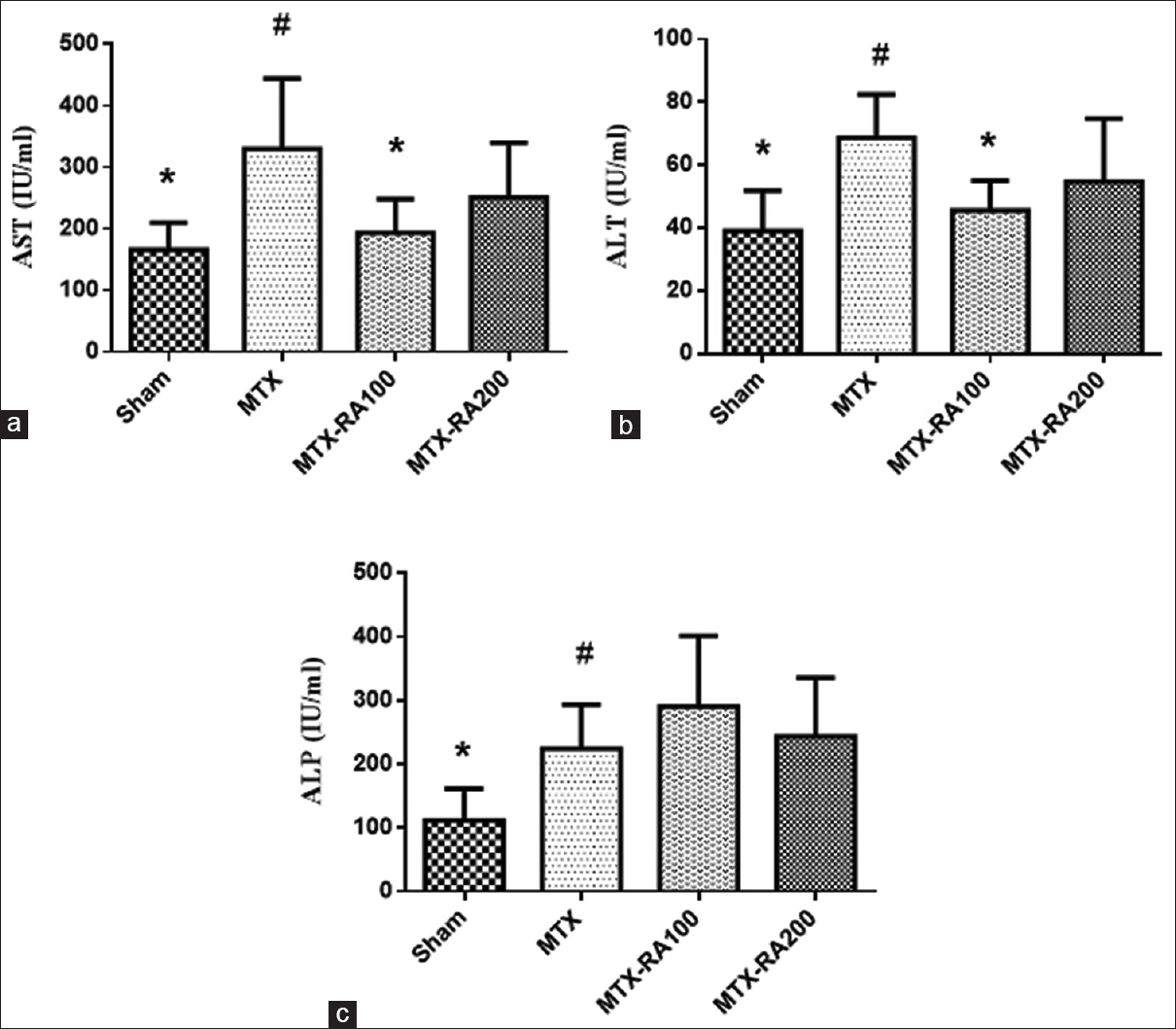
- Effects of Rosmarinic acid on liver function tests in methotrexate-induced hepatorenaltoxicity. Data were expressed mean ± SEM (n = 8). Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. * Significant difference compared to MTX group (P < 0.05), # significant difference compared to Sham group (P < 0.05)
Effects of RA on renal oxidative stress parameter and antioxidant enzymes
The level of antioxidant enzymes and renal MDA levels are illustrated in Table 1. MDA, as the final product of lipid peroxidation and oxidative stress index, significantly increased in the sham group (p < 0.05). Receipt of RA in both groups significantly reduced the MDA level (p < 0.05). GSH level, and GPX and CAT activities in groups receiving MTX decreased compared with the sham group, but this decline only was significant in CAT (p < 0.05). The level of GSH and activity of all other antioxidants (CAT & GPX) in groups receiving RA as pretreatment showed an increase; except that the CAT activity in group 4 showed a significant increase (p < 0.05).
| Sham | MTX | MTX-RA100 | MTX-RA200 | |
|---|---|---|---|---|
| MDA (nmol/g) | 45.78±3.46a | 62.08±2.38b | 40.26±3.38a | 36.01±2.42a |
| GSH (mg/g) | 10.40±1.14 | 9.23±1.02 | 11.76±0.93 | 9.35±0.76 |
| CAT (U/g) | 40.91±3.76a | 25.74±3.59b | 38.61±3.82 | 41.24±4.37a |
| GPX (U/g) | 122.18±26.45 | 77.66±4.04 | 107.03±4.38 | 102.59±6.50 |
Data were expressed mean±SEM (n=8). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test. asignificant difference compared to MTX group (P<0.05), bsignificant difference compared to Sham group (P<0.05)
Effects of RA on liver oxidative stress parameter and antioxidant markers
Liver oxidative stress markers are reported in Table 2. There was no significant difference in MDA level in both sham and MTX groups, but pretreatment with RA significantly decreased the liver MDA compared with the MTX group (p < 0.05). GSH level and CAT activity in the MTX group significantly declined compared with the sham group (p < 0.05). Treatment with RA only at 200 mg/kg dose significantly increased the GSH (P < 0.05).
| Sham | MTX | MTX RA100 | MTX RA200 | |
|---|---|---|---|---|
| MDA (nmol/g) | 62.08±2.38 | 67.08±2.42 | 47.74±2.09a | 45.70±3.38a |
| GSH (mg/g) | 9.84±0.61a | 6.49±0.34b | 8.66±0.75 | 8.80±0.49a |
| CAT (U/g) | 48.09±2.15a | 37.00±3.17b | 32.22±2.60b | 35.91±2.33b |
| GPX (U/g) | 103.99±4.48 | 104.34±4.72 | 103.94±3.86 | 89.81±2.78 |
Data were expressed mean±SEM (n=8). Data were analyzed by one way ANOVA followed by Tukey’s post hoc test. asignificant difference compared to MTX group (P<0.05), bsignificant difference compared to Sham group (P<0.05)
Effect of RA on renal histopathology
The amount of tubular necrosis, leukocyte infiltration, eosinophilic casts, and glomerular damage of renal tissue is reported in Table 3. Receiving MTX caused renal tissue destruction; consequently, all of the above cases had significantly increased tissue destruction compared with the sham group (p < 0.05). Receipt of RA as a pretreatment could considerably decline the amount of renal tissue destruction and this was observed in all of above cases except eosinophilic casts in RA 200 mg/kg group, and the amount of glomerular damage in the group received RA 100 mg/kg did not reveal any significant decrease [Figure 3].
| Sham | MTX | MTX-RA100 | MTX-RA200 | |
|---|---|---|---|---|
| Tubular necrosis | 1.61±0.15a | 2.78±0.24b | 1.69±0.1a | 2.08±0.08a |
| Leukocyte infiltration | 1.13±0.10a | 2.16±0.16b | 1.39±0.09a | 1.53±0.17a |
| Eosinophilic casts | 1.26±0.11a | 2.10±0.87b | 1.61±0.10a | 1.84±0.06 |
| Glomerular damage | 5. 70±0.19a | 1.32±0.22b | 0.84±0.1 | 0.69±0.09a |
Data were expressed mean±SEM (n=8). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test. a significant difference compared to MTX group (P<0.05), bsignificant difference compared to Sham group (P<0.05)

- Effect of rosmarinic acid on kidney histopathological changes in methotrexate induced nephrotoxicity model. (Stained by hematoxylin and eosin with magnified × 400). a: Sham, b: MTX, c: MTX-RA 100, d: MTX-RA 200
Effects of RA on liver histopathology
Liver tissue changes in groups explored in this study are illustrated in Table 4. The tissue parameters examined in this study are as follows: necrosis; degeneration; and cellular vacuolization. MTX significantly increased the amount of these three parameters compared with the sham group (p < 0.05). RA in its two doses could significantly decrease the amount of degeneration and cellular vacuolization compared with the MTX group (p < 0.05). Although RA decrease necrosis of liver cells, this decline was not significant [Figure 4].
| Sham | MTX | MTX-RA100 | MTX-RA200 | |
|---|---|---|---|---|
| necrosis | 0.74±0.16a | 1.29±0.21b | 0.94±0.12 | 0.88±0.10 |
| degeneration | 0.82±0.21a | 1.60±0.22b | 0.90±0.12a | 0.79±0.08a |
| cellular vacuolization | 0.70±0.22a | 1.41±0.26b | 0.74±0.06a | 0.74±0.11a |
Data were expressed mean±SEM (n=8). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test. a significant difference compared to MTX group (P<0.05), bsignificant difference compared to Sham group (P<0.05)
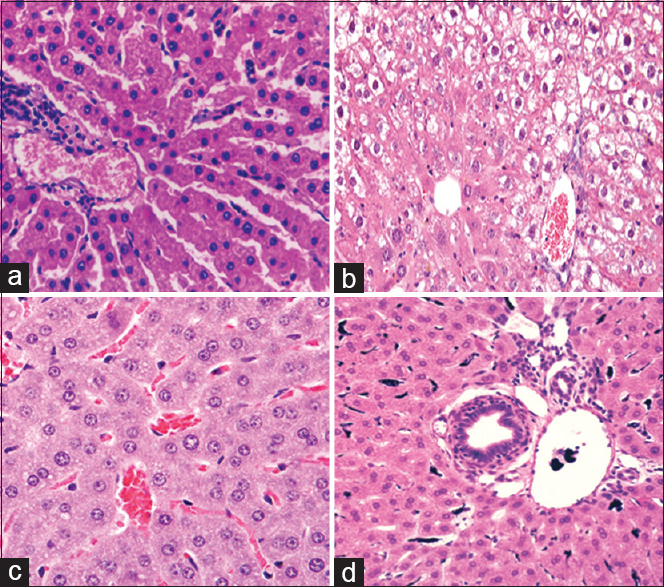
- Effect of rosmarinic acid on liver histopathological changes in methotrexate induced hepatotoxicity model. (Stained by hematoxylin and eosin with magnified × 400). a: Sham, b: MTX, c: MTX-RA 100, d: MTX-RA 200
Discussion
The injection of 20 mg/kg MTX for 9 days caused functional disorder in both liver and kidney. According to the results of biochemical examinations, MTX caused the increase of oxidative stress and the decrease of antioxidant enzymes in liver and kidney tissues. Also, histopathological damage and lesions were found in the liver and kidney. Receiving RA as a pretreatment with two doses of 100 mg/kg and 200 mg/kg for 12 days led to improvement of liver and kidney functions, reinforcement of antioxidant system, and decrease of hepatic and renal tissue destructions. Thus, it can be concluded that RA has decreased the hepatotoxicity and nephrotoxicity induced by methotrexate. Oxidative stresses induced by MTX cause the increase of lipid peroxidation product, MDA, No level, MPO activity, and then decrease of catalase activity and glutathione level that finally lead to hepatic and renal injuries.[11819] Our study revealed an increase in serum level of AST, ALT, ALP induced by injection of 20 kg/mg MTX for 9 days. The increase in the level of these tests indicates liver function disorder. The results of previous studies also showed that MTX leads to liver function injuries; consequently, it increases the level of its functional tests.[17182021] In our study, serum urea level in the group treated with MTX has increased that it indicated the induced nephrotoxicity. The previous studies have also reported the increase of functional tests induced by injection of MTX.[1721] According to current studies, the use of antioxidants such as thymoquinone, diosmin, and berberine has hopefully decreased the level of renal and hepatic function tests in animals treated with MTX.[201721] A significant decrease in urea, creatinine, AST, ALT was observed in the group treated with 100 mg/kg rosmarinic acid. RA played an important role in the decline of various disease injuries such as sepsis, hepatotoxicity induced by using acetaminophen, renal ischemia-reperfusion in an animal model.[102223] MTX caused an increase of oxidative stress in hepatic and renal tissues; as a result, the increase of MDA level in these organs, and the decrease in the antioxidant defensive system; especially renal CAT, and hepatic GSH which significantly increased. Recent studies conducted on toxicity induced by treatment with MTX in an animal model have reported the increase of oxidative stress in different organs, and the decrease of antioxidant enzymes.[2017212425] In some studies, berberine, diosmin, thymoquinone, and silymarin directly decrease the lesions by removing the ROS and indirectly by increasing the number of injured organ antioxidants due to their antioxidant properties.[20172126] RA possibly reduced hepatotoxicity and nephrotoxicity induced by MTX by the decrease of lipid peroxidation due to its antioxidant and anti-inflammatory properties, and RA ameliorates the abnormalities of these organ.
MTX causes renal and hepatic tissue injuries by, producing oxidative stress, ROS, caspase 3, and apoptotic markers attributed to the properties of anti-cancer drug.[18192728] Histopathological analysis of liver and kidney tissue showed that the use of 20 mg/kg MTX causes the increase of tubular necrosis, leukocyte infiltration, eosinophilic casts, glomerular damage in kidney tissue, and necrosis, cellular vacuolization, degeneration in liver tissue that MTX likely causes tissue necrosis, increases inflammation and edema, and leads to the mentioned tissue changes by producing ROS. Most of previous studies have also reported the histopathological renal and hepatic changes induced by use of MTX.[2017212728] Some antioxidants decrease the tissue injuries induced by treatment with MTX via reducing oxidative conditions.[20172128] In our study, RA caused the decrease of tissue inflammation, cellular necrosis, and degeneration in liver and kidney. RA prevented the formation of free radicals; therefore, the injury to DNA and its necrosis will decrease. Furthermore, RA directly decreases the inflammation and bleeding in tissues because of its anti-inflammatory activity.[2223] Rosmarinic acid decreased painful peripheral neuropathy that may be due to its anti-inflammatory, and anti-apoptotic properties.[29] RA had beneficial effects in on memory deficits induced by cerebral ischemia that it may be related to antioxidant, antiapoptotic activities and anti-inflammatory and synaptogenic mechanisms of RA.[30] The effect of rosmarinic acid in the bile duct ligation model indicated beneficial effects against inflammation, oxidative stress, and fibrosis. Thus, rosmarinic acid might be beneficial in treating cholestatic liver injury.[31] In addition, RA decreased TGF-β1, CTGF expression that this improves biochemical parameter and histopathological. Different mechanisms exists for liver toxicity and RA improves liver damage in the different models.[32] In Domitrovic ´et al's study, RA ameliorated acute liver toxicity by induction of the Nrf2/HO-1 pathway that it in this model RA reduced inflammation, hepatic oxidative, and apoptosis in liver.[33] RA inhibited tumor growth by suppression of inflammatory cytokines, angiogenic factors, and NF-kB p65 in the H22-xenografts model,[34] and Renal damage induced by gentamicin is ameliorated by lycopene and rosmarinic acid alone or combined.[35] Natural antioxidants such as curcumin, garlic, olive leaves, rosemary, and pomegranate prevent kidney disease and renal dysfunctions in animal models. Furthermore, these antioxidants might be beneficial for humans.[36] Thus, RA may reduce tissue injuries induced by MTX due to its anti-inflammatory and anti-apoptotic properties.
Conclusion
The findings of this study show that RA in an animal model with its antioxidant and anti-inflammatory properties can play an important role in the prevention of renal and hepatic injuries induced by MTX. RA and foods which contain this compound may help in the treatment of acute kidney and liver toxicity.
Financial support and sponsorship
Dezful University of Medical Sciences and the research center of Razi Herbal Research Center of Lorestan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to appreciate the help from all the researchers at Dezful University of Medical Sciences and the research center of Razi Herbal Research Center of Lorestan University of Medical Sciences.
References
- Curcumin ameliorates methotrexate-induced nephrotoxicity in rats? Adv Pharmacol Sci. 2013;2013:387071. doi: 10.1155/2013/387071
- [Google Scholar]
- Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694-703.
- [Google Scholar]
- β-glucan ameliorates methotrexate-induced oxidative organ injury via its antioxidant and immunomodulatory effects. Eur J Pharmacol. 2006;542:170-8.
- [Google Scholar]
- Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2-and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther. 2007;320:229-35.
- [Google Scholar]
- Prevalence and risk factors of methotrexate hepatoxicity in Asian patients with psoriasis. World J Hepatol. 2013;5:275-80.
- [Google Scholar]
- Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294-300.
- [Google Scholar]
- Rosmarinic acid ameliorates acute liver damage and fibrogenesis in carbon tetrachloride-intoxicated mice. Food Chem Toxicol. 2013;51:370-8.
- [Google Scholar]
- Nephroprotective activities of rosmarinic acid against cisplatin-induced kidney injury in mice. Food Chem Toxicol. 2014;66:321-8.
- [Google Scholar]
- Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell. 2011;43:392-7.
- [Google Scholar]
- Antioxidant effect of rosmarinic acid against renal ischemia-reperfusion injury in rat; a histopathological study. Ann Res Antioxid. 2016;1:e24.
- [Google Scholar]
- SD.[30] Microsomal lipid peroxidation. Methods in enzymology. 52: Elsevier 1978:302-10.
- A biochemical and cytochemical study of peroxidase activity in roots of Pisum sativum: II. Distribution of enzymes in relation to root development. J Exp Bot. 1978;29:983-91.
- [Google Scholar]
- [13] Catalase in vitro. Methods in enzymology. 105: Elsevier 1984:121-6.
- Role of endothelium-related mechanisms in the pathophysiology of renal ischemia/reperfusion in normal rabbits. Circ Res. 1996;79:1031-8.
- [Google Scholar]
- The effect of alpha lipoic acid on rat kidneys in methotrexate induced oxidative injury. Eur Rev Med Pharmacol Sci. 2015;19:2132-9.
- [Google Scholar]
- Diosmin attenuates methotrexate-induced hepatic, renal, and cardiac injury: A biochemical and histopathological study in mice? Oxid Med Cell Longev. 2017;2017:3281670. doi: 10.1155/2017/3281670
- [Google Scholar]
- Effects of pentoxifylline and alpha lipoic acid on methotrexate-induced damage in liver and kidney of rats. Environ Toxicol Pharmacol. 2015;39:1122-31.
- [Google Scholar]
- Beneficial effects of Chrysin against Methotrexate-induced hepatotoxicity via attenuation of oxidative stress and apoptosis. Mol Cell Biochem. 2014;385:215-23.
- [Google Scholar]
- Hepatoprotective effect of berberine against methotrexate-induced liver toxicity in rats. Biomed Pharmacother. 2018;97:233-9.
- [Google Scholar]
- Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats? Mediators Inflamm. 2015;2015:859383. doi: 10.1155/2015/859383
- [Google Scholar]
- Does rosmarinic acid treatment have protective role against sepsis-induced oxidative damage in Wistar Albino rats? Hum Exp Toxicol. 2016;35:877-86.
- [Google Scholar]
- Effects of rosmarinic acid on acetaminophen-induced hepatotoxicity in male Wistar rats. Pharm Biol. 2017;55:1809-16.
- [Google Scholar]
- Protective effects of alpha-lipoic acid on methotrexate-induced oxidative lung injury in rats. J Invest Surg. 2018;31:107-13.
- [Google Scholar]
- The effect of vitamin E and L-carnitine against methotrexate-induced injury in rat testis. Turk J Med Sci. 2015;45:517-25.
- [Google Scholar]
- Therapeutic effects of silymarin and naringin on methotrexate-induced nephrotoxicity in rats: Biochemical evaluation of anti-inflammatory, antiapoptotic, and antiautophagic properties. J Food Biochem. 2017;41:e12398.
- [Google Scholar]
- Protective effect of peroxisome proliferator activator receptor (PPAR)-α and-γ ligands against methotrexate-induced nephrotoxicity. Immunopharmacol Immunotoxicol. 2014;36:130-7.
- [Google Scholar]
- Pomegranate reverses methotrexate-induced oxidative stress and apoptosis in hepatocytes by modulating Nrf2-NF-γB pathways. J Nutr Biochem. 2013;24:2040-50.
- [Google Scholar]
- Rosmarinic acid attenuates development and existing pain in a rat model of neuropathic pain: An evidence of anti-oxidative and anti-inflammatory effects. Phytomedicine. 2018;40:59-67.
- [Google Scholar]
- Rosmarinic acid prevents against memory deficits in ischemic mice. Behav Brain Res. 2016;297:91-103.
- [Google Scholar]
- Hepatoprotective activities of rosmarinic acid against extrahepatic cholestasis in rats. Food Chem Toxicol. 2017;108:214-23.
- [Google Scholar]
- Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action? Biomed Pharmacother. 2019;112:108600. doi: 10.1016/j.biopha.2019.108600
- [Google Scholar]
- Rosmarinic acid ameliorates acute liver damage and fibrogenesis in carbon tetrachloride-intoxicated mice. Food and Chem Toxicol. 2013;51:370-8.
- [Google Scholar]
- Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-γB signaling in H22 tumor-bearing mice. J Pharmacol Sci. 2016;132:131-7.
- [Google Scholar]
- Effect of Lycopene and Rosmarinic acid on gentamicin induced renal cortical oxidative stress, apoptosis, and autophagy in adult male albino rat. Anat Rec. 2017;300:1137-49.
- [Google Scholar]
- Prevention of nephropathy by some natural sources of antioxidants. Yangtze Med. 2017;1:235-66.
- [Google Scholar]







