Translate this page into:
Deceased Donor Renal Transplantation: A Single Center Experience
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Deceased donor kidney transplant are still not common across India. This study was done to assess various measures taken at a single center level to increase organ donation rate and to analyse the outcomes of transplants performed from these donors.
Methods:
All deceased donor renal transplants performed from November 2011 to February 2017 were analysed for patient and death censored graft survival, rate of delayed graft function, rate of rejection and mortality. Kaplan Meir analysis for Survival Curves was used.
Results:
Organ donation rate at our center improved from one donation every alternate year in 2004 to a peak of 44 donations in 2017. Patient survival was 93.42%, 89.44%, 85.53%, and death censored graft survival was 94.07%, 88.21%, and 82.86% at 1, 2 and 3 years respectively. Mean duration of hemodialysis pre transplantation was 34.6 ± 27.43 months.
Conclusions:
This study has shown that steps taken at a single center level alone can also significantly improve organ donation rates. Employment of dedicated professionals including transplant surgeons and coordinators, developing a protocol-based approach for referral, and early counseling in triage along with regular audits can help to establish deceased donor program with acceptable outcomes elsewhere in the country.
Keywords
Brain death
deceased donor transplant
organ donation
Introduction
In India, approximately 175,000 patients are added each year to the pool of End Stage Renal Disease (ESRD). The prevalence of stage 5 chronic kidney disease (CKD) in India has been reported at 0.8%[1] and the incidence of ESRD has been pegged at 150–232 cases per million population.[23] Only 10% of these receive Renal Replacement Therapy (RRT) and only 2.4% of these undergo Renal Transplant (RT) with majority of them receiving kidney from a living donor.[2] The rest remain on dialysis in absence of a suitable donor. As maintenance dialysis is considerably more expensive than transplantation, a deceased donor renal transplant (DDRT) program can provide organs to these patients. However, the deceased–donation rate remains abysmally low at 0.65 Population per Million (PMP) per year in our country.[4] There is a dire need to improve awareness regarding organ donation amongst both the general public as well as health care professionals.[56] Some of the Indian states have been able to increase the rate of organ donation in an impressive way with the help of government support, non-governmental organizations (NGOs) and cooperation amongst various government and private sector hospitals, but lack of Intensive Care Unit (ICU) facilities for taking care of potential donors, and low public awareness regarding brain death have been quoted as significant hurdles to deceased organ donation in the rest of the country. However, there are no studies on measures taken at a single center level to increase the organ donation rate without substantial support from the government. This study depicts the impact of various measures taken at the hospital level alone to improve organ donation rate over the last decade and describes the outcomes of renal transplants performed from these donors.
Methods
Patient files and electronic medical records database were reviewed from November 2011 to February 2017 to analyze the trend in organ donation. The changes implemented during this period included:
-
Sensitization of Hospital administration, neurophysicians, and neurosurgeons for promoting organ donation.
-
Creation of posts of transplant coordinators
-
Monthly audits, development of protocol for referral of patients for organ donation (transplant coordinators were informed of all patients with serious irreversible head injury and Glasgow Coma score of less than 5)
-
Development of multi organ transplant program
-
Active identification of organ donors in the triage area
-
Accepting marginal donors including utilizing organs from donors with cardiac arrest.
These changes were implemented across different time periods and impact of each on organ donation activity was noted. Follow up data of recipients was available for analyzing outcomes from November 2011 to February 2017. 152 DDRT were performed during this period. Kaplan Meir analysis was done to measure patient and death censored graft survival.
Organ allocation and recipient selection
Our organ sharing is still in its infancy and organ allocation across transplant programs is not yet standardized, hence recipients were allocated organs as per the local allocation criteria [Table 1] mainly based on waiting period on dialysis. One point was given for each month of waiting on dialysis. The time to start accruing points on waiting list started from date of registration or date of start of dialysis, whichever was later. Extra points were awarded to patients with the conditions as highlighted in the table. All patients presenting to the renal transplant clinic were registered for deceased donor transplantation regardless of the state of domicile, but they were counseled to stay within a 6 hours distance from the hospital once they were on the active waiting list. Patients with co-morbid conditions such as pre-existing coronary artery disease (CAD) were required to undergo any intervention suggested by the cardiologist prior to transplant. Hepatitis C positive patients were required to complete therapy and obtain negative viral load prior to being considered on active waiting list. Patients were duly informed of the risks and benefits of an Extended Criteria Donor (ECD) kidney versus continuing dialysis during pre-operative counseling and they were given the option to either accept the kidney or continue to wait for a Standard Criteria Donor (SCD) kidney.
| Condition | Points awarded |
|---|---|
| Children <15 years of age | 20 |
| Living donors presenting with end stage renal disease | 50 |
| 1st degree relations of deceased donor | 40 |
| Failed 1st transplant from a living related donor provided non compliance as a cause of graft loss is ruled out | 20 |
| Sensitized patient as defined by the presence of any Anti - HLA antibody with MFI >5000 | 30 |
| Following patients were screened by Panel Reactive Antibodies (PRA) and/or single antigen Donor Specific | |
| Antibody (DSA) profiling | |
| Failed organ transplant | |
| History of multiple transfusions | |
| History of multiple pregnancies | |
| Positive x match with a living/deceased donor | |
| Familial kidney disease whose other sibling has been transplanted and no suitable living donor | 10 |
| Marginal kidneys would be allocated to recipients more than 50 years of age. Criteria for marginal kidneys include - donor age more than 50 years in the presence of long standing hypertension, diabetes, H/O cerebrovascular accident, or terminal creatinine more than 3.0, or donor age more than 60 years in the absence of any of the above comorbidities. | |
| Simultaneous organ recipients (Pancreas Kidney, Liver Kidney) would receive priority in allocation of one of the two kidneys. |
Immunosuppressive regimen
All patients received induction with rabbit-anti-thymocyte globulin (r-ATG) (3 mg/kg in 3 divided doses) or Basiliximab 20 mg on day 0 and day 4, and Hydrocortisone 500 mg intravenously at the time of induction of anesthesia. Maintenance immunosuppression consisted of prednisolone (0.4 mg/kg/day tapered to 5 mg/day by 3 months post-transplant and continued thereafter), mycophenolate mofetil (MMF) (1.5–2 g/day), and calcineurin inhibitors (CNI) [cyclosporine {CsA} (8 mg/kg/day or tacrolimus, 0.2 mg/kg/day) as starting dose]. Target trough (C0) concentrations for cyclosporine were 250–350 ng/ml during the first month, 150–250 ng/ml between 1 and3 months after transplantation, and 50–150 ng/ml thereafter. Tacrolimus dosing was adjusted to achieve target C0 concentrations of 10–15 ng/ml during the first month after transplantation, 8–10 ng/ml from 1 to 3 months and 5–8 ng/ml thereafter. All patients were started on tacrolimus, which was changed to cyclosporine in 6% of patients that is 9 out of 152 patients due to uncontrolled diabetes despite adequate treatment with insulin and oral hypoglycemics or inability to achieve therapeutic tacrolimus levels.
All patients received prophylaxis against cytomegalovirus (CMV) infection (Valgancyclovir, dose adjusted as per creatinine clearance) for 3 months, and pneumocystis jiroveci pneumonia (trimethoprim/sulfamethoxazole (TMP/SMX 80/400 mg once a day for 12 months). Graft survival and patient survival was calculated using Kaplan Meir analysis, and both graft and patient survival were compared in patients with DGF and without DGF, and in patient induced with ATG vs Basiliximab.
Donor selection criteria
All except one donor were selected from our institute and both kidneys were utilized at our center. Kidneys were preserved in histidine–tryptophan–ketoglutarate or UW solution. An age limit of 60 years was set as an exclusion criterion in the early period. Over the last couple of years, the donor acceptance criteria were expanded to include donors at extremes of age, as well as those with controlled sepsis, diabetes mellitus, hepatitis C, circulatory death (DCD), hypertension, and acute kidney injury. The first DCD transplant was done in August 2011 and donors more than 60 years were accepted from 2013 onwards. ECD kidneys were offered to elderly recipients only, age >60 years. A frozen section was done at the time of harvesting to decide whether to do dual kidney transplant versus implanting both kidneys in separate recipients.
Results
The number of donations increased significantly over this period. Yearly trends in the organ donation rate along with each intervention have been depicted in Figure 1. The first Deceased donor kidney transplant at our center was performed on 23 July 1998. From 1998 to 2008, only 10 kidney transplants happened from 5 cadaveric donations, one each in 1998, 2001, 2005, and two in 2006. In 2005, a separate department was created to cater to the needs of transplant patients. The number of organ donations increased after the first intervention in the form of a symposium on organ donation in 2008 to 2 donations in 2008, and 4 donations in the subsequent years of 2009 and 2010. After the initiation of multi organ transplant in 2011, along with appointment of a part time transplant coordinator, the donation rate increased to 5 in 2011 and 7 in 2012. The number of transplants of other abdominal and thoracic organs has shown significant increase since 2011 and this has been depicted in Table 2.

- Serial trend in the number of donors annually with each intervention
| Year | Kidney | Liver | Pancreas | Heart | Lung | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DDTx | Shared | DDTx | Shared | DDTx | Shared | DDTx | Shared | DDTx | Shared | |
| 2011 | 10 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2012 | 14 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2013 | 20 | 0 | 4 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 2014 | 14 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| 2015 | 52 | 0 | 6 | 9 | 2 | 0 | 1 | 0 | 0 | 0 |
| 2016 | 46 | 2 | 6 | 3 | 5 | 0 | 1 | 1 | 0 | 0 |
| 2017 | 80 | 0 | 10 | 4 | 3 | 0 | 1 | 5 | 1 | 1 |
| 2018 | 64 | 0 | 14 | 2 | 7 | 0 | 0 | 2 | 0 | 0 |
| 2019 | 59 | 0 | 5 | 3 | 3 | 0 | 1 | 1 | 0 | 0 |
| TillMar 2020 | 18 | 0 | 1 | 1 | 4 | 0 | 0 | 0 | 0 | 0 |
| Total | 377 | 2 | 52 | 24 | 25 | 0 | 5 | 10 | 1 | 1 |
Appointment of a full-time transplant coordinator in 2012 led to increase in donations to 10 in 2013 and 7 in 2014. However, the biggest jump in rate of organ donation happened in 2015, when the number of transplant coordinators was increased to three and counseling of the families of potential donors was started in the triage area which led to 24 donations in 2015 and 23 in 2016. The institute was designated as Regional Organ and Tissue Transplant Organization (ROTTO) in March 2016 whose support and activities along with relaxation in the donor acceptance criteria and improved resuscitation in the triage area led to another significant increase with 44 donations in 2017 and 36 donations in 2018.
152 patients underwent renal transplantation from 81 donations during this period. The donor and recipient demographics have been given in Tables 3 and 4, respectively. Eight patients were lost to follow up out of the total of 152 patients. A total of 6 dual kidney transplants have been performed so far, but all of them were done after the end of study period for recipients, that is February 2017 thus not included in the analysis.
| Mean Donor Age | 34.2517±15.16 years |
| Median donor age | 32 years |
| Range of Donor age | Maximum=75 years |
| Minimum=11 years | |
| Donor Sex | Male=59 (72.8%) |
| Female=22 (27.2%) | |
| Cause of death | Road Traffic Accident=71 |
| Cerebrovascular Accident=7 | |
| Fall From Height=3 | |
| Type of Donation | Donation after Brain Death (DBD) = 74 (91.3%) |
| Donation after Cardiac Death (DCD) (All Uncontrolled) = 7 (8.7%) |
| Mean Recipient age | 39±11.36 years |
| Median Recipient age | 38 years |
| Range of Recipient age | Maximum=66 years |
| Minimum=13 years | |
| Recipient sex | Male=89 (58.5%) |
| Female=63 (41.5%) | |
| Cause of Renal Failure | Chronic Glomerulonephritis=91 |
| Diabetes Mellitus type 2=15 | |
| Diabetes Mellitus type 1=3 | |
| ADPKD=9 | |
| Renal Stone Disease=3 | |
| Miscellaneous=31 | |
| Mean duration of HD pre-transplantation | 34.617±27.43 months |
| Mean Cold Ischemia Time (CIT) | 4.9317±2.7 hrs |
| Delayed graft | Yes=53 (34.87%) |
| Function (DGF) | No=99 (65.13%) |
| Induction | ATG=107 |
| Immunosuppression | Basiliximab=44 |
| No induction=1 | |
| CNI used | Tacrolimus=143 (94%) |
| Cyclosporine A=9 (6%) |
Patient survival of kidney transplant recipients was 93.42%, 89.44%, 85.53%, and death censored graft survival was 94.07%, 88.21%, and 82.86% at 1, 2, and 3 years, respectively [Figures 2 and 3]. Both patient and graft survival were lower in patients who had DGF versus those who did not have DGF, but the difference was not found to be statistically significant (p value 0.45 and 0.78, respectively) [Figures 4 and 5].
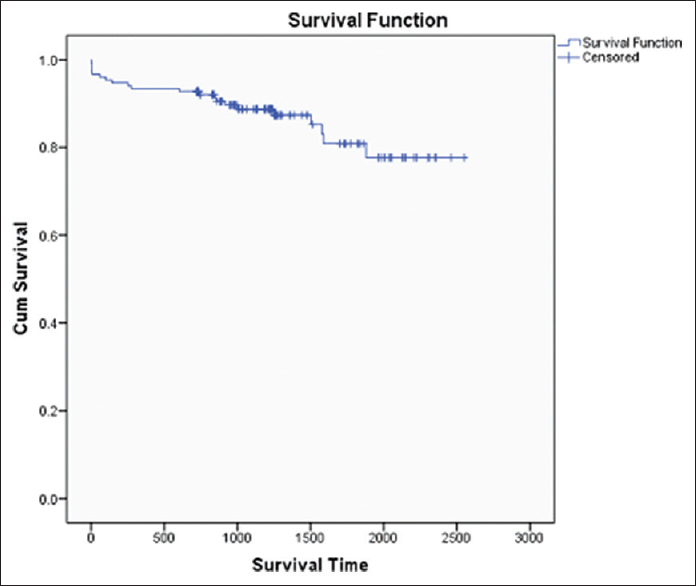
- Patient Survival Curve
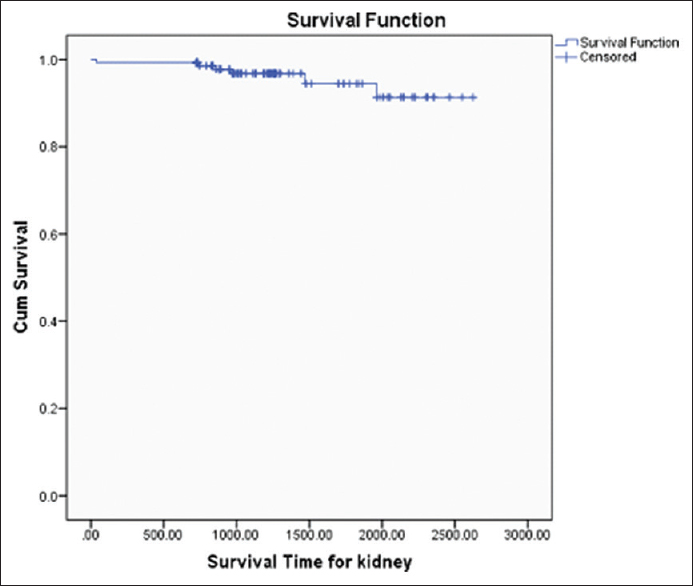
- Graft Survival Curve
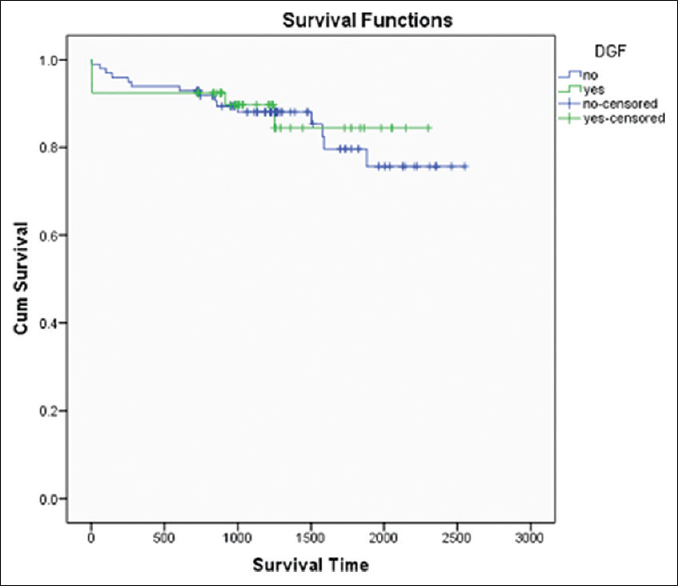
- Patient Survival Curve with and without DGF
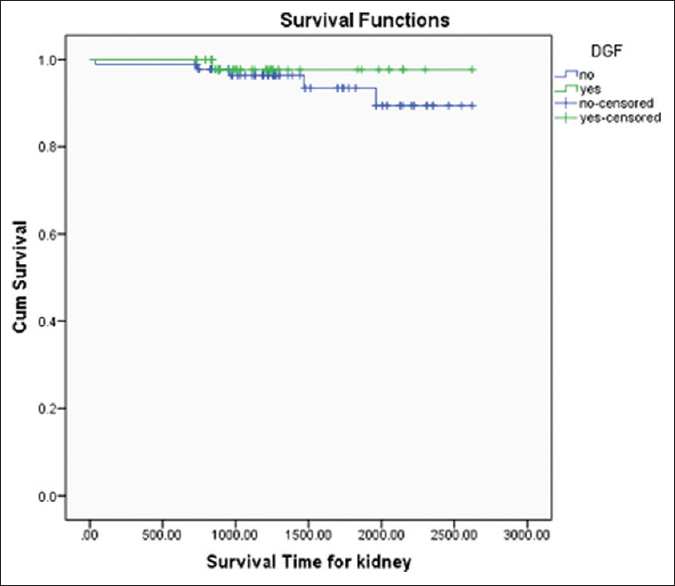
- Graft Survival Curve with and without DGF
Anti-Thymocyte Globulin (ATG) was used in 107 patients, Basiliximab was used in 44 patients, and one patient did not receive any induction due to financial constraints. Basiliximab was used for patients either deemed to be low immunological risk such as elderly and diabetics or patients with high risk of post-transplant infectious diseases such as Hepatitis C or recent history of treated tuberculosis. Patient survival and graft survival was compared amongst the patient induced with ATG versus Basiliximab as shown in Figures 6 and 7, respectively, and the difference was not found to be significant (p value 0.51 and 0.31, respectively).
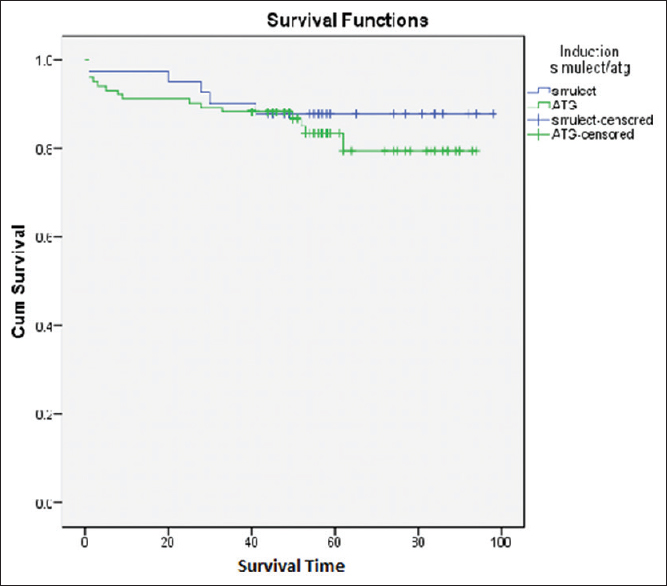
- Patient Survival curve ATG vs Simulect
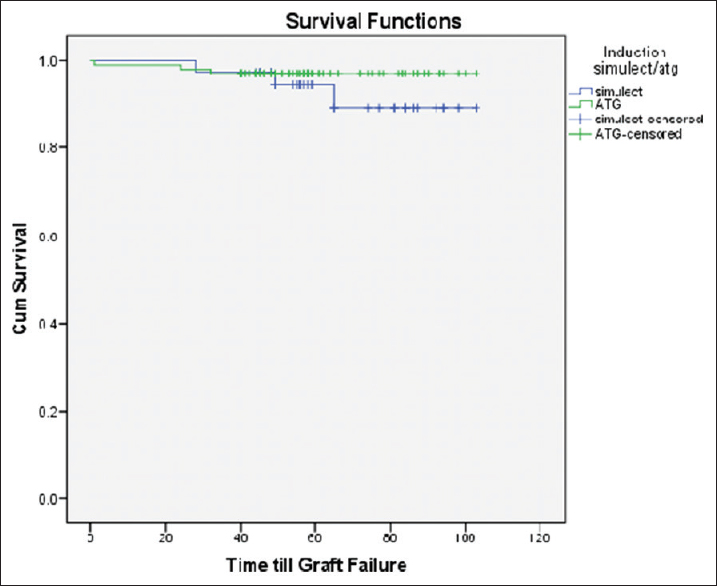
- Graft Survival curve ATG vs Simulect
A total of 21 (13.8%) patients died during this period, 14 due to bacterial sepsis, 2 due to fungal infection, 3 due to CMV disease, and 2 due to acute myocardial infarction.
Twenty-seven (17.76%) patients had biopsy proven acute rejection. Out of these, acute cellular rejection was present in 12 patients, mixed AMR+ACR in 2 patients, acute antibody mediated rejection was noted in 10 patients, and chronic AMR was noted in 3 patients.
Mean Serum creatinine (mg/dl) at 1 month, 1 year, and at last follow up was 1.3517 ± 0.53, 1.17 ± 0.41, and 1.21 ± 0.47, respectively.
Discussion
The rate of organ donation in India is still low despite its need and tremendous potential. However, the rate of deceased organ transplants has increased across the country over the last few years with concerted efforts across some of the states. A list of measures taken by various Indian centers and State of Tamil Nadu to improve organ donation rates has been enumerated in Table 5. A similar success was witnessed at our center over the last 10 years, resulting in a significant increase in organ donation. This manuscript analyses the role of various measures and their impact on the organ donation rates.
| Centre/State Name | Measures Taken |
|---|---|
| Tamil Nadu[16] | 1. Positive steps taken by the state government |
| 2. Public awareness on organ donation | |
| 3. Public Private Partnership | |
| 4. Reducing the cost of transplant | |
| 5. Appointment of a central coordinator | |
| 6. Involvement of non governmental organisation, “Multi Organ Harvesting And Network” foundation | |
| Army Hospital Research | 1. Use of kidneys from marginal donors |
| and Referral[14] | 2. Appointment of transplant coordinator |
| 3. Coordinated team effort willing to go extra mile | |
| Institute of Kidney | 1. Increased public awareness. |
| Diseases and Research | 2. Identification of potential donors in general hospitals. |
| Centre, Ahmedabad[15] | 3. Legislation to procure deceased donor organs. |
| 4. Reduction of cost of transplantation. | |
| 5. Early brain death identification and certification | |
| 6. Establishment of rapid response team. | |
| 7. Improvement in transportation for organ retrieval. | |
| 8. Trained transplant co-coordinator. | |
| 9. Improved hospital infrastructure. | |
| 10. Inclusion of expanded criteria donors (ECD). | |
| 11. Positive steps by the government NGOs. |
The top management of any institution plays a very important role in promotion of any activity and a full support for organ donation from the management resulted in monthly meetings and audits with all stakeholders, where case history of each potential donor which was missed was discussed threadbare and corrective steps for future were taken. Protocols were made for identification, timely referral and management of potential brain-dead organ donors. The neurosurgical team was involved and one of their senior consultants was nominated to supervise the referral process.
While ongoing public education programs were held by the ROTTO team to raise general awareness regarding organ donation, one of the most effective steps was appointment of a full-time transplant coordinator. The role of transplant coordinator cannot be undermined as they perform the key duties of donor identification, counseling and provide support to the grieving families. The donations in our hospital doubled in 2015, when the number of transplant coordinators was increased to three. It has been shown in other studies as well that employment of transplant coordinators significantly increases the number of organ donations in the region.[7]
Initiation of counseling regarding organ donation in triage area was another step taken in 2015 which led to a rapid increase in the donation rate. The earlier practice was to counsel relatives of patients admitted in the ICU. However, it was realized that the most grievously hurt patients with irreversible brain damage would never make it to the ICU due to shortage in ICU beds. This is possibly true for all major hospitals in the country. There were ethical concerns about counseling in triage area and this decision was taken after a lot of discussion amongst the major stakeholders. The counseling was done after explaining regarding the grave condition of the patient and futility of further care and the talk about organ donation was initiated once the family realized the futility of continuing further care. Early counseling outside the ICU care is practiced in other areas of the world as well and has been to shown to improve consent and organ utilization rates in Spain.[8]
Utilizing organs from the marginal donors also helped us to expand donor pool for the kidney recipients. Marginal donors in this study included donors with documented infections which were appropriately treated before donation, raised terminal creatinine, history of diabetes, hypertension, older age, donors with hepatitis C infection. Kidneys from elderly donors were used for elderly recipients or as dual kidney transplants. Kidneys from Hepatitis C positive donors were used for Hepatitis C positive recipients but now there is evidence in literature to support transplant of these kidneys into naïve patients as well in the era of Directly Acting Antivirals (DAAs).[9]
Our institute started the Donation after Circulatory Death (DCD) program in 2012. The documented incidence of cardiac arrest in ICU patients is 22.7 per 1000 admissions.[10] This constitutes a pool of potential organ donors which is largely untapped in our country. Unlike in the West, all DCD donations in our hospital have been Maastricht category IV as there are still no guidelines regarding withdrawal of life support in terminally sick patients in India. Previously published experience of DCD transplants from our institute has shown acceptable results.[11] DCD donors contributed to about 10% of renal transplants performed during this period. The establishment of a separate department for transplantation also played an important role in increasing the organ donation activity. With the presence of dedicated manpower and resources, it was possible to convert many challenging situations like DCD, marginal donors into successful outcomes.
The outcomes of the transplants at our center were slightly better compared to the outcomes reported by other Indian centers. Infections remained the most common cause of mortality, unlike the Western data where cardiovascular causes are the leading reason of mortality.[12] In India, only a few individual centers have reported their outcomes after deceased donor transplants.
Data from Madras Medical College, Chennai of 173 recipients of deceased donor kidneys showed a patient and death censored graft survival at 1 year were 80 and 82.6% and at 5 years were 76 and 80%, respectively. Forty-one patients died, 75% of them in the first post–transplant year. Sepsis and cardiovascular disease were the most common causes of death. Twenty-two percent patients had acute rejection.[13]
According to the data published by the Army Hospital for Research and Referral out of a total of 44 renal transplants were done with organs retrieved from 35 deceased donors between August 1998 and April 2011. Post-transplant, 15 patients (34%) had DGF [due to Acute Tubular Necrosis (ATN) in 7 patients, acute cellular rejection in 5, and antibody-mediated rejection in 2 patients.[14]
Outcomes of 160 deceased donor renal transplants done at Institute of Kidney Disease and Research Center (IKDRC), Ahmedabad, showed a patient survival of 79.58%, 76.7%, 74.8%, and graft survival was 92.4%, 87.9%%, and 87.9% at 1, 2, and 3 years, respectively. As in this study, infections were responsible for death in majority (27/36, 75%) of the cases. Totally, 14% had biopsy proven acute rejection (n = 22). DCD donors formed 14.3% of total donations in their study.[15]
Limitations of the study
It was a retrospective study and the effect of individual interventions on the organ donation rate cannot be ascertained exactly, as there was overlap and individual interventions might have impacted the organ donation program at a later stage.
Conclusions
This study has shown that steps taken at a single center level alone can also significantly improve organ donation rates even without substantial help from the government. Employment of dedicated professionals including transplant surgeons and coordinators, developing a protocol-based approach for referral, and early counseling in triage along with regular audits can help to establish deceased donor program with acceptable outcomes elsewhere in the country.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Epidemiology and risk factors of chronic kidney disease in India – results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol. 2013;14:114.
- [Google Scholar]
- The incidence of end-stage renal disease in India: A population-based study. Kidney Int. 2006;70:2131-3.
- [Google Scholar]
- IRoDAT Certified [Internet]. Available from: http://www.irodat.org/img/database/grafics/01_worldwide-actual-deceased-organ-donors-2017.png
- Attitude and awareness towards organ donation in western India. Ren Fail. 2015;37:582-8.
- [Google Scholar]
- Knowledge and attitude toward organ donation among health-care professionals in a rural town in India. Saudi J Kidney Dis Transpl. 2018;29:671-9.
- [Google Scholar]
- Impact of introducing full-time in-house coordinators on referral and organ donation rates in Rio de Janeiro Public Hospitals: A health care innovative practice. Transplant Proc. 2016;48:2396-8.
- [Google Scholar]
- How Spain reached 40 Deceasd organ donors per million population. Am J Transplant. 2017;17:1447-54.
- [Google Scholar]
- Transplantation of kidneys from HCV-positive donors: How to best use a scarce resource. J Am Soc Nephrol. 2017;28:3139-41.
- [Google Scholar]
- The incidence of cardiac arrest in the intensive – care unit: A systematic review and meta-analysis. J Intensive Care Soc. 2019;20:144-54.
- [Google Scholar]
- A Single-center experience of kidney transplantation from donation after circulatory death: Challenges and scope in India. Indian J Nephrol. 2017;27:205-9.
- [Google Scholar]
- Trends in the causes of death among kidney transplant recipients in the United States (1996-2014) Am J Nephrol. 2018;48:472-81.
- [Google Scholar]
- Deceased donor renal transplantation: A single center experience. Indian J Nephrol. 2017;27:4-8.
- [Google Scholar]
- Deceased donor renal transplantation at army hospital research and referral: Our experience. Indian J Urol. 2013;29:105-9.
- [Google Scholar]
- Deceased donor organ transplantation: A single center experience. Indian J Nephrol. 2011;21:182-5.
- [Google Scholar]
- State of deceased donor transplantation in India: A model for developing countries around the world. World J Transplant. 2016;6:331-5.
- [Google Scholar]







