Translate this page into:
Two Cases of Vasculitis with Renal Vein Thrombosis
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We present a series of two cases of ANCA associated Vasculitis (AAV) presenting as Pulmonary Renal syndrome with associated renal vein thrombosis. Although there are enough evidences suggesting association of venous thrombosis with AAVs, the incidence of renal vein thrombosis is rare. Renal vein thrombosis should be ruled out in cases where there is delay in recovery of renal function in patients with AAV. Positive laboratory values for anti-Proteinase-3 (PR3) and anti-Myeloperoxidase (MPO) ANCA in the cases that presented as Rapidly Progressive Glomerulonephritis, helped in early initiation of treatment with complete recovery of Renal function.
Keywords
ANCA
renal vein thrombosis
vasculitis
Background
Antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis is characterized by an inflammatory process causing occlusion of small vessels thereby involving multiple organ system. Patients with ANCA associated vasculitis (AAV) may have a wide spectrum of systemic symptoms caused by involvement of Lungs, Kidneys, Central Nervous System. Venous thromboembolic events (VTE) are known to be associated in AAV as in the WeCLOT study, which was a prospective observational study of patients with Wegener's granulomatosis.[1] This showed an incidence of VTE of 7 per 100 patients years, which was significantly higher than that described for normal population cohorts. Weidner et al.[2] reported that 13 of 105 patients with AAV had thromboembolic events during the period of active disease. Seven of these had pulmonary emboli and 3 had iliac and/or caval vein thrombosis. However, none of the 13 patients had typical risk factors for VTE. Another retrospective study confirmed an increased risk of developing VTEs at 1.8 per 100 patient years overall, increasing to 6.7 during active disease, which also appeared independent of classic risk factors.[3] The occurrence of Renal Vein thrombosis is a rare entity in AAV and none of the studies mentioned above had cases associated with of Renal Vein thrombosis.
Case 1
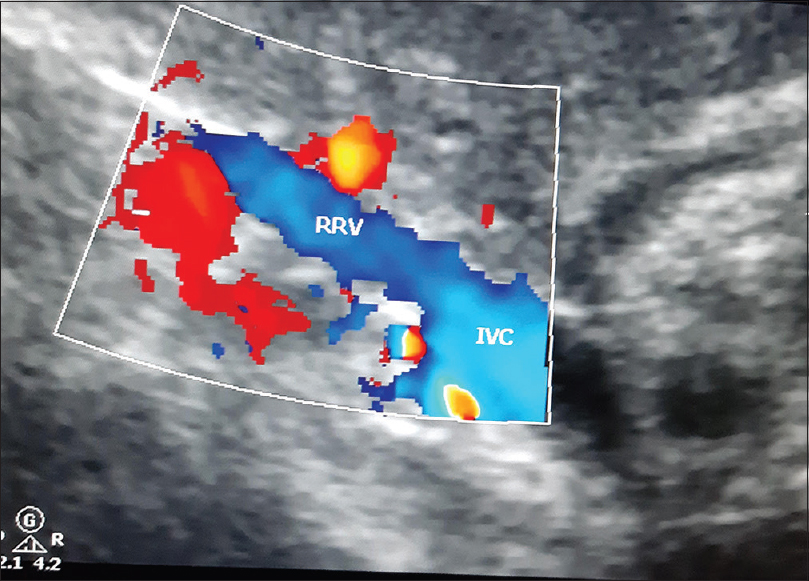
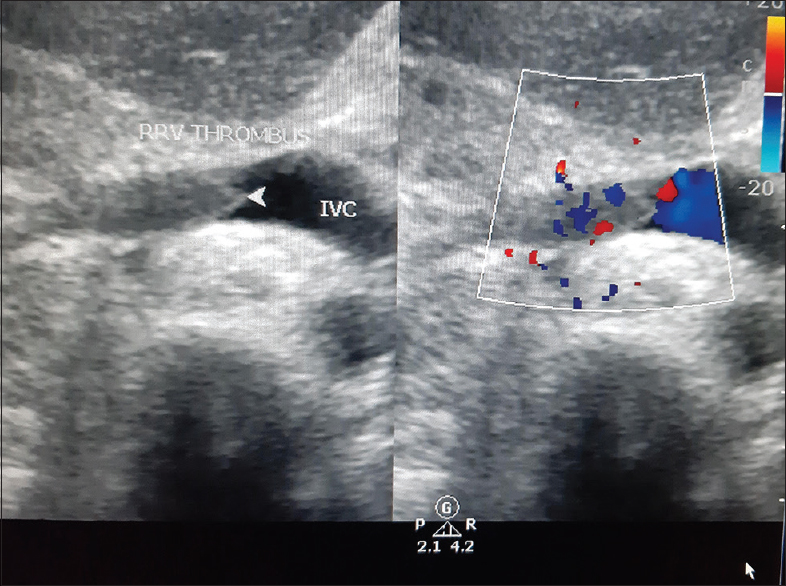
A 28-year-old, 10 week primigravida presented with complaints of generalised edema of one week duration, with recent increase in swelling of both lower limb associated with pain. On examination she was afebrile, normotensive with normal respiratory rate and both lower limbs showed pitting edema. Homans' sign was positive with tenderness in left calf. Systemic examination was unremarkable. Initial investigations showed normal renal function (S. creatinine of 1.1 mg/dl) with Hb of 11.2 g/dl which gradually worsened over a period of 4-5 days and Hb dropped to 7.0 g/dl with leukocytosis (23,000/mm3), normal platelets 2,04,000/mm3 and rising levels of serum creatinine to 2.38 mg/dl. Liver function tests were normal. Urine routine showed 1+ protein, 6 WBC and 43 RBC with RBC casts. 24 hour urinary protein was 1729 mg. ANCA was positive with cytoplasmic pattern by IF with anti PR3 antibody elevated to 63 IU/ml by ELISA, with negative MPO-ANCA. ANA by ELISA was negative, Lupus anticoagulant, anti-CCP, APLA, Rheumatoid factor, ASO titre, Anti Thrombin activity, Protein C, Protein S, Factor V Leiden mutation analysis and viral markers were negative, C3 was 1.39 g/L, C4 was 27 mg/dl (normal). Chest X-ray showed minimal bilateral pleural effusion with left upper zone consolidation of possible Vasculitic origin. Renal ultrasonography with Doppler study showed bilateral normal sized kidneys with grade 1 echogenicity with normal CMD with right renal vein thrombus [Figure 1] extending to Inferior Vena cava and bilateral infra renal venous thrombosis. Bilateral Lower limb Venous Doppler showed left popliteal vein thrombosis and bilateral posterior tibial vein thrombosis. Uterus had a single live foetus of 10 weeks duration. 2D ECHO showed normal LV function of 60% with no LV clot or vegetation. Hence, she was diagnosed as having anti pr3 positive Granulomatosis with polyangitis. She had to undergo medical termination of pregnancy in view of teratogenic nature of treatment. She was given induction therapy with high dose IV methylprednisolone 1 gm for 3 days and started on anti-coagulants initially unfractionated heparin and then warfarin. Later six doses of IV cyclophosphamide in low dose of 0.5 gm/m2 were given every 15 days. Low doses of Cyclophosphamide were given to avoid drug toxicity. She responded well to the treatment with improvement in clinical symptoms, and subsequent reports suggesting complete recovery of renal function, resolution of proteinuria and microscopic haematuria. Birmingham Vasculitis Activity Score[4] was used for monitoring the response which showed improving trend. She is currently maintained on oral prednisolone, mycophenolate mofetil and Warfarin. Follow up evaluation showed recanalized renal vein [Figure 2] and normal venous doppler. Plan of care was discussed with the patient and the need of drug modification in the event of a pregnancy was explained.

- Ultrasonography study showing Right Renal Vein Thrombosis
- USG Doppler study shows Recanalization of Renal vein after treatment
Case-2
A 64-year-old lady presented with worsening shortness of breath, nausea and anorexia for 1 week. She had complaints of backache and dry cough for last 6 months following which she was diagnosed as a possible case of sputum negative pulmonary tuberculosis and was empirically started on Anti-TB medicines. On examination she was afebrile, normotensive with tachypnoea. Systemic examination showed bilateral asymmetrical crepitations with rhonchi. Hb was 7.2 g/dl, TLC was 8630/mm3 with normal platelets with creatinine levels elevated to 5.31 mg/dl. Liver function and coagulation profile was normal. Urine Routine and microscopy showed 2+ proteinuria, WBC 3 and RBC 17. On further work up, ANCA by IF showed perinuclear pattern with MPO >100. Anti PR3-ANCA, APLA, Anti-GBM, Anti-cardiolipin Ab, Lupus Anticoagulants tests, AFP, CEA, CA 125, CA 19-9 were negative.
Ultrasonography of Abdomen showed normal sized kidneys with grade 1 echogenicity with normal CMD with normal liver, spleen and pancreas. No pelvic mass was seen. Renal Doppler [Figure 3] showed right renal vein thrombosis. Chest X-ray was suggestive of opacities in both lower zones, MRI spine showed no evidence of nerve root compression or changes suggestive of Potts spine. She was diagnosed as having anti MPO-AAV with renal vein thrombosis. Anti-Tb treatment was stopped and she was started on IV Methyl Prednisolone 1 gm for 3 days followed by IV cyclophosamide low dose 0.5 gm/m2 every 15th day and oral anti-coagulants. Post initiation of treatment, her pulmonary infiltrates resolved, renal function gradually recovered, haematuria and proteinuria resolved. Treatment response was monitored using Birmingham Vasculitis Activity Score. Currently she is on Oral Prednisolone, Mycophenolate Mofetil and Warfarin.
- USG Doppler study showing Right Renal Vein Thrombosis
Discussion
The patients as mentioned presented as a case of Rapidly Progressive Renal failure with Pulmonary Renal Syndrome with evidence of an underlying possible Vasculitis. On evaluation a diagnosis of ANCA associated small vessel vasculitis was made, with strongly positive anti PR3 ANCA in case 1 and anti MPO ANCA in case 2 by ELISA. Renal biopsy was deferred in both the cases as the patients were started on anti-coagulants in view of associated renal vein thrombosis finding at the time of presentation. Wlodek et al. reported a case with bilateral renal vein thrombosis in AAV in a male patient.[5] Kiykym A et al. reported a case of a female patient with extensive iliac and inferior vena cava thrombosis with AAV.[6] There may be pre-existing thrombophilic factors that may contribute to thrombosis in individual cases,[7] however the primary reason for predisposition to thrombosis seems to be the inflammatory process in the vessel wall progressing to endothelial injury and dysfunction in such cases.[8] Venous thromoembolic events are associated with high disease activity in AAV cases, as described by Stassen et al. Incidence of VTE was more during active disease; 6.7/100 person- years as compared to 1/100 person-years in those without active disease. Occurrence of VTE was lesser in patients with Wegner's Granulomatosis and in those with ANCA specificity for PR3.[3] However, hypercoagulable state has been reported in patients with AAV in Remission. Marc Hillhorst et al. reported that patients with AAV in remission had elevated levels of Endogenous Thrombin Generation Potential (ETP) and Factor VIII levels as compared to age and sex matched healthy individuals, rendering them in a more procoagulable state. High ETP values were found in both anti-MPO AAV patients and anti-PR3 AAV patients.[9] Berden et al. investigated the role of anti-plasminogen antibodies in AAV and concluded that patients with anti-plasminogen antibodies had significantly higher percentages of glomeruli with fibrinoid necrosis and cellular crescents, the patients had more severe renal dysfunction as compared to patients without these antibodies.[10] In the above mentioned cases presentation of rapidly progressive renal failure with renal vein thrombosis with strongly positive laboratory values suggesting AAV helped in timely initiation of treatment ensuring complete recovery of renal function in both the cases. In the above discussed cases, though there was no delay in recovery of renal functions due to early diagnosis, the case series aims to highlight the rare incidence of renal vein thrombosis in AAV. Therefore, screening for the same may be considered in cases where there is a delay in recovery of renal function or poor response to treatment in AAV patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Brief communication: High incidence of venous thrombotic events among patients with Wegener granulomatosis: The Wegener's clinical occurrence of thrombosis (WeCLOT) study. Ann Intern Med. 2005;142:6206.
- [Google Scholar]
- Thromboembolic events as a complication of antineutrophil cytoplasmic antibody associated vasculitis. Arthritis Rheum. 2006;55:1469.
- [Google Scholar]
- Venous thromboembolism in ANCA-associated vasculitis—incidence and risk factors. Rheumatology. 2008;47:5304.
- [Google Scholar]
- The utility of urinalysis in determining the risk of renal relapse in ANCA associated vasculitis. Clin J Am Soc Nephrol. 2018;13:2517.
- [Google Scholar]
- Anti-neutrophil cytoplasmic antibody vasculitis presenting with bilateral renal vein thrombosis. Clin Kidney J. 2012;5:22931.
- [Google Scholar]
- Extensive iliac and inferior vena cava thrombosis associated with ANCA positivity in a patient with type IV crescentic glomerulonephritis. Clin Nephrol. 2000;54:E78.
- [Google Scholar]
- Pathological haemostasis and “prothrombotic state” in Behcet's disease. Thromb Res. 2002;105:125-33.
- [Google Scholar]
- Patients with antineutrophil cytoplasmic antibodies associated vasculitis in remission are hypercoagulable. J Rheumatol. 2013;40:2042-6.
- [Google Scholar]
- Anti-plasminogen antibodies compromise fibrinolysis and associate with renal histopathology in ANCA- associated vasculitis. Am Soc Nephrol. 2010;21:216979.
- [Google Scholar]







