Translate this page into:
Spontaneous Cerebrospinal Fluid Rhinorrhea in End Stage Renal Disease
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We report a case of spontaneous cerebrospinal fluid (CSF) rhinorrhea in a patient on maintenance hemodialysis. There was no previous history of trauma or surgery. Secondary hyperparathyroidism due to progression of chronic kidney disease (CKD) and a rise in intracranial pressure resulted in spontaneous cerebrospinal fluid rhinorrhea. He underwent endoscopic endonasal repair with theco-peritoneal shunt; CSF leak stopped completely and the patient is doing well on one year follow up.
Keywords
Cerebrospinal fluid
chronic kidney disease
hemodialysis
hyperparathyroidism
Introduction
Cerebrospinal fluid (CSF) rhinorrhea occurs due to communication between intracranial subarachnoid space and sinonasal mucosa. It presents as a flow of clear fluid from the nose. This rhinorrhea is usually unilateral and is aggravated by straining or stooping down. The most common cause is head injury causing fracture of base of skull.[1]
Spontaneous CSF rhinorrhea is more common in obese middle aged women.[2] It also occurs in chronic kidney disease (CKD) due to secondary hyperparathyroidism leading to cortical bone erosion or as a consequence of benign intracranial hypertension (also called pseudotumorcerebri) which is a syndrome of markedly elevated intracranial pressure resulting from fluid overload and increased cerebral blood flow in the absence of intracranial mass, inflammation, or obstruction.[3]
Case Report
A 69-year-old male, a known case of systemic hypertension and end stage renal disease (ESRD), on maintenance hemodialysis thrice weekly for two years, presented to our center with history of clear fluid discharge from the left nostril. It was continuous, worsened in sitting position and bending forwards. He had accelerated hypertension (BP: 200/100 mmHg) with fundus showing grade I hypertensive retinopathy. Neurological examination was normal. There was no history of trauma or any surgery in the head and neck region. Laboratory investigations are shown in Table 1. Nasal discharge analysis revealed glucose of 71 mg%. There were no leucocytes and no bacterial or fungal growth was seen in culture [Table 2]. Glucose concentration of more than 30 mg/dl without any blood contamination in the nasal fluid was suggestive of CSF in the nasal fluid. CSF pressure was 28 cm of water.
| Laboratory investigations | Results |
|---|---|
| Hemoglobin | 9.5 gm/dl |
| Total leucocyte count | 6500/cu mm |
| Platelet count | 150,000/cumm |
| Plasma glucose (Random) | 77 mg/dl |
| Serum creatinine | 6.63 mg/dl |
| Serum Sodium | 137.4 mmol/L |
| Serum Potassium | 4.6 mmol/L |
| Serum Calcium | 8.4 mg/dl |
| Serum Phosphorous | 6.66 mg/dl |
| Serum Vitamin D | 40.39 nmol/L |
| Serum Intact Parathyroid hormone (iPTH) | 186 pg/ml |
| Laboratory investigations | Results |
|---|---|
| Glucose | 71 mg/dl |
| Albumin | 15.9 mg/dl |
| Total count | 0 cells/cu mm; inflammatory cells - nil and no organisms seen |
| Culture | No growth after 5 days of incubation |
| Fungal smear | No fungal elements |
CT cisternogram [Figure 1] detected a leak through a small bony defect of size 3.8 mm, in the left lateral wall of pterygoid recess of the left sphenoid sinus, between foramen rotundum and vidian canal (pterygoid canal). There was contrast opacification within left sphenoid sinus and posterior ethmoid sinus. CT brain showed evidence of partial empty-sella indicating raised intracranial pressure [Figure 2].

- CT cisternogram showing a defect of size 3.8mm in the lateral recess of the left sphenoid sinus between foramen rotundum and vidian canal and contrast opacification within the left sphenoid sinus
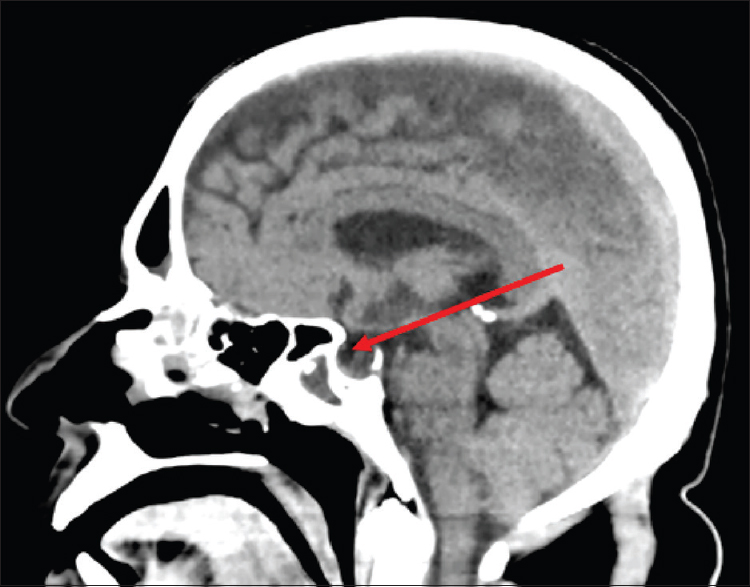
- CT Brain showing evidence of partial empty sella
ENT and Neurosurgery team did endoscopic endonasal repair of the defect with theco-peritoneal shunt under general anesthesia. Fluorescein dye was injected into the thecal sac, and a trans-pterygoid CSF leak was detected. Pterygoid plate was drilled to gain access to the lateral recess of left sphenoid sinus [Figure 3a]. Margins of the defect were freshened and the fat harvested from the right thigh was plugged into the defect to stop the leakage of CSF. Fascia lata graft was placed over the fat plug and secured with fibrin glue. Flourescein was also found to be leaking from multiple sites in the left cribriform plate and roof of ethmoids on the left side. Fat was plugged in the leaking sites after elevating the mucosa and reinforced with fascia lata [Figure 3b] and fibrin glue over the fascia, both in the region of cribriform plate/ethmoid roof and in the lateral wall of the sphenoid sinus. There was no further leakage of CSF as indicated by fluorescein. The patient improved dramatically after the surgery. He was advised sedentary activity for two weeks. He had no symptoms suggestive of recurrence of CSF rhinorrhea on follow up for twelve months.
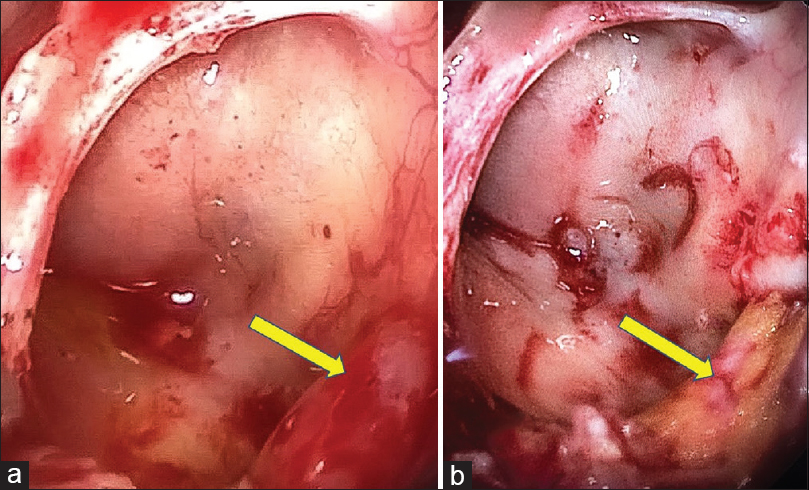
- Endoscopy findings: Trans-pterygoid approach - (a). Defect in the lateral recess of left sphenoid sinus, (b). Defect sealed with fat (yellow) and reinforced by fascia lata
Discussion
Cerebrospinal fluid (CSF) rhinorrhea is a condition in which there is a perforation of the skull base that results in communication between the subarachnoid space and surrounding extracranial spaces leading to leakage of CSF. The potential leak sites are the cribriform plate (35%), ethmoid (26%), sphenoid (18%), frontal sinus (10%), posterior ethmoid sinus (9%) and inferior clivus (2%).[2] Our patient had a leakage from the left sphenoid sinus , cribriform plate and posterior ethmoid sinus.
History of a clear, colorless, usually unilateral nasal discharge strongly suggests CSF rhinorrhea. Other presenting symptoms are due to the underlying etiology. It is classically positional in nature and exacerbated by dependent head positioning (i.e., “reservoir sign”).[4] Patients may note salty or sweet taste in the mouth after nasal drainage. Glucose concentration in the nasal fluid, if more than 30 mg/dl without any blood contamination strongly suggests CSF rhinorrhea and the absence of glucose rules out CSF rhinorrhea. Even though glucose estimation is non-specific, it is the most readily available method of diagnosis.[5] Our patient had glucose concentration of 71 mg/dL in the nasal discharge. Beta-2 (β-2) transferrin is a protein mostly present in CSF and its detection is a gold standard laboratory test for the diagnosis of suspected CSF rhinorrhea;[5] this test is not widely available. High-resolution computed tomography and magnetic resonance cisternography are complementary to each other and are the investigations of choice to identify the site of leak.[5]
Meningitis is the most common (10 to 36%) and serious complication of CSF rhinorrhea, which can result in significant morbidity and mortality.[4] Organisms causing meningitis secondary to CSF rhinorrhea are found in upper respiratory tract – most commonly by Streptococcus pneumoniae, followed by Neisseria meningitidis and Haemophilus influenza; Pseudomonas aeuroginosa and Escherichia coli are rare.[6]
A study from National Inpatient Sample (NIS) database in the United States published in 2017 identified 1.8% of CKD among CSF rhinorrhea cases (n = 1541).[4] The risk factors for developing CSF rhinorrhea in a case of CKD are impaired vitamin D metabolism, phosphate retention, raised parathyroid hormone and frequent hemodialysis.[3] Our patient was on maintenance hemodialysis thrice weekly since two years with hypophosphatemia and raised iPTH level.
CSF Rhinorrhea and CKD
The decline in glomerular filtration rate in CKD leads to less excretion of phosphate in urine causing increase in plasma phosphate and decrease in serum calcium concentration. These metabolic disturbances lead to secondary hyperparathyroidism which in turn causes increased resorption of calcium and phosphorous from bone which is the major reservoir of calcium. These changes constitute chronic kidney disease – mineral and bone disorder (CKD – MBD).[7] The subperiosteal bone resorption in hyperparathyroidism is responsible for the cortical bone erosion associated with CKD. Spontaneous CSF leakage probably results from enlargement of the foramina at the lamina cribrosa due to calcium mobilization from bones and pseudotumorcerebri. Our patient with CKD developed CSF rhinorrhea due to secondary hyperparathyroidism as evidenced by high iPTH (intact parathyroid hormone level 186 pg/ml).
Idiopathic or acquired intracranial hypertension (pseudotumorcerebri) results in increased hydrostatic pressure at anatomically weakened sites of skull which causes dura to herniate into sella turcica and fills it with CSF. This will compress pituitary, giving the appearance of empty sella and cause spontaneous CSF leak. It may be associated with menigoencephalocele formation.[3] Our case had high CSF pressure and a partial empty sella on CT-scan; it also contributed to the development of CSF rhinorrhea along with secondary hyperparathyroidism.
Endoscopic repair of the leak with intrathecal fluorescein localization of leak and lumbar drain has success rate of 98% but without fluorescein localization and lumbar drain, the success rate is only 88.5%.[2]
Localization of CSF leak is done by intrathecal injection of dye (fluorescein 5%) or a radio isotope and placing pledgets of cotton in olfactory slit, middle meatus, spheno ethmoidal recess and near eustachian tube and examining pledgets for dye or radio activity. The precise location of site and the size of fistula is the keystone for successful endoscopic closure. The closure techniques depend on the size and location of cranial defect. The three forms of grafting are the underlay, overlay and combined.[4] The overlay grafting using fluorescein localization was done in this case.
Conclusion
Spontaneous CSF rhinorrhea is a very rare complication seen in patients with CKD. Routine monitoring of serum calcium, phosphorous, parathyroid hormone (PTH) and vitamin D, along with proper medical measures are essential in patients with CKD to minimize the effects of CKD – MBD.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Spontaneous CSF rhinorrhea our experience. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl 2):271-5.
- [Google Scholar]
- Endoscopic repair of CSF rhinorrhea: An institutional experience. Iran J Otorhinolarngol. 2016;28:39-43.
- [Google Scholar]
- Spontaneous cerebrospinal fluid rhinorrhea associated with chronic renal failure. Neurol Med Chir (Tokyo). 2001;41:313-7.
- [Google Scholar]
- An analysis of patients treated for cerebrospinal fluid rhinorrhea in the United States from 2002 to 2010. J NeurolSurg. 2017;78:18-23.
- [Google Scholar]
- Endoscopic management of cerebrospinal fliuid rhinorrhea. Asian J Neurosurg. 2018;11:183-93.
- [Google Scholar]
- Cerebrospinal fluid rhinorrhea and recurrent meningitis. Clin Infect Dis. 1993;17:364-8.
- [Google Scholar]
- Chronic kidney disease – Mineral bone disorder. In: Taal MW, Cherlow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM, eds. Brenner and Rector's The Kidney (9th ed). Elsevier; 2012. p. :2021-58.
- [Google Scholar]







