Translate this page into:
Recurrent, Atypical Anti-Glomerular Basement Membrane Disease
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Anti-glomerular basement membrane disease (GBM) (Goodpasture's disease) typically presents with acute manifestations of rapidly progressive glomerulonephritis often accompanied by lung haemorrhage. Anti-GBM disease is usually monophasic. However, atypical presentations with indolent renal involvement are being increasingly recognized. Herein we report a patient who presented with lung haemorrhage, minimal renal involvement, and negative result for serum anti-GBM antibody, while immunofluorescence examination of the renal biopsy provided the diagnosis leading to the institution right treatment with excellent response. Interestingly, he had presented 10 years earlier with lung hemorrhage, more significant renal involvement clinically and histologically, with positive serum anti-GBM antibody. The present case is intended to increase our awareness regarding the variable presentations of anti-GBM disease, such as with negative serology and recurrence of anti-GBM disease. The presentation of anti-GBM nephritis with non-proliferative, non-crescentic glomerulonephritis is also highlighted. The possible explanations for negative serum anti-GBM antibody are explored with a brief review of literature.
Keywords
Atypical anti-GBM disease
Goodpasture's disease
lung haemorrhage
rapidly progressive glomerulonephritis
recurrent anti-GBM disease
Introduction
Anti-glomerular basement membrane disease (anti-GBM disease) usually manifests with rapidly progressive glomerulonephritis (RPGN) often accompanied by pulmonary haemorrhage (pulmonary-renal syndrome). It is typically monophasic and does not relapse. However, reports of variable clinical manifestations with anti-GBM disease are emerging. Antibody negative Goodpasture's disease is being recognized increasingly. We present herewith a case spanning over a decade, with different renal phenotypic presentations at different times.
Case Report
A 30-year-old male presented with fevers, cough, hemoptysis, and increasing shortness of breath of 5 days duration. He had not noticed any urinary symptoms. He was a current smoker.
On current admission, patient was febrile and mildly breathless. CT-chest showed diffuse alveolar opacities [Figure 1]. BP was 120/80 mm Hg. Urinary antigens for Pneumococci and Legionella were negative. Respiratory viral antigen testing for Influenza A and B, parainfluenza, respiratory syntitial virus, adenovirus, and human metapneumovirus was negative. Serum creatinine was 0.7 mg/dl. Urinalysis showed 10–100 RBCs × 106/L; urine protein/creatinine ratio was normal initially and mildly elevated on day 3 at 17 mg/m mol (normal:<12 mg/m mol). Anti-GBM by fluoro-enzymatic immune assay was negative (2.2 u/ml; normal <7) and anti-neutrophil cytoplasmic antibodies (ANCA) were negative. He was started on antibiotics for presumed community acquired pneumonia.
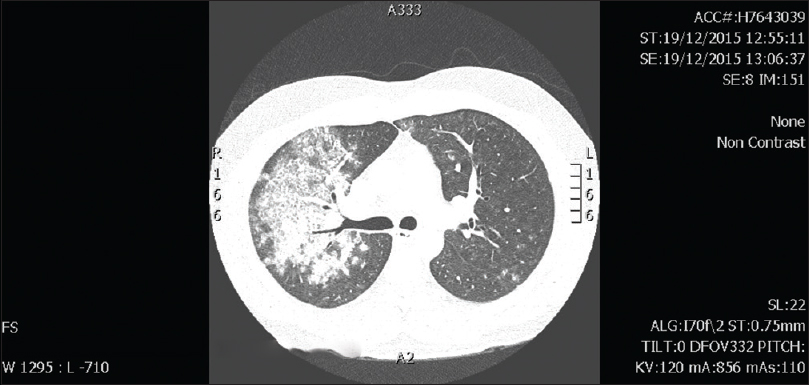
- CT Chest: Diffuse parenchymal opacities on current presentation
The past history was significant for the diagnosis of anti-GBM disease in Feb. 2007 in another state when he had presented with hemoptysis and had positive anti-GBM antibody by ELISA (48 U/ml; normal <20). He had microscopic glomerular haematuria with serum creatinine was 1 mg/dl. Renal biopsy had revealed 84 glomeruli with epithelial cellular crescents in 15 glomeruli with linear staining IgG and C3c on immunofluorescence (IF). He had received plasma exchange (PEX) for 2 weeks with corticosteroids and cyclophosphamide for 6 months resulting in complete resolution of all markers of disease activity and had remained well.
During the current admission, the breathlessness, cough, and hemoptysis worsened whilst on antibiotics for 72 h. In view of microscopic hematuria and trace proteinuria, and acknowledging that serum anti-GBM antibody can be sometimes negative despite active disease, renal biopsy was performed on day 3 which revealed 16 glomeruli, with no crescents or proliferative changes [Figure 2]. On IF, there was linear staining of GBM with IgG (+++) and C3c, with linear kappa and lambda light chains consistent with anti-GBM disease. The patient was started on IV Methyl Prednisolone, PEX and rituximab. The respiratory symptoms resolved dramatically within 24 h of starting methyl prednisolone. Patient received IV methyl prednisolone 500 mg daily for 3 days, 6 sessions of PEX and one dose of Rituximab 500 mg (as he had previously received Cyclophosphamide), with continued oral prednisolone for 6 months. Appropriate Pneumocystis Jeroveci cover with Sulfamethoxazole/trimethoprim was also instituted. Renal function continues to be normal with no proteinuria or hypertension 2 years later. There are no chest symptoms. The patient has quit smoking.
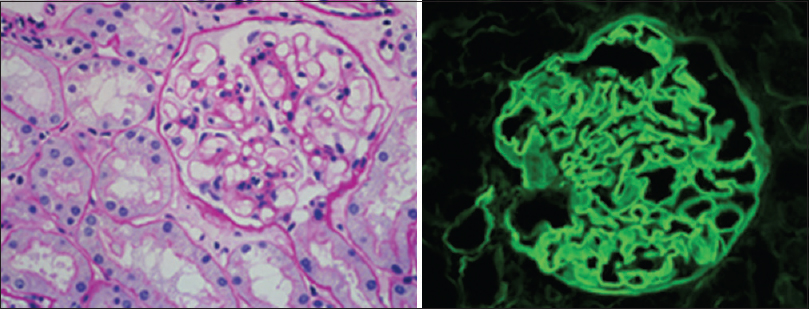
- KIDNEY BIOPSY on current presentation: Light Microscopy: The Glomerulus is essentially normal, and shows no proliferative or crescentic glomerulonephritis. (PAS stain ×40) Immunofluorescence: Section shows linear IgG along the glomerular basement membrane. (IgG ×40)
Initially the patient presented with a diagnostic challenge with infective symptoms and was started on antibiotics for presumed infective pneumonia. He continued to deteriorate rapidly with worsening haemoptysis and shortness of breath. There were no urinary symptoms and he was normotensive. Serum anti-GBM antibody and ANCA were negative. Microscopic hematuria was noted, but its value was debatable in the absence of significant proteinuria, hypertension, or renal impairment. However, he continued to worsen with his respiratory symptoms and reconsidering his past history, a renal biopsy was performed.
Discussion
Anti-GBM disease is rare, with a prevalence of 0.5–1 per million per year. Non-collagenous domain of alpha-3 chain (α-3 NC1 domain) of Collagen IV of the glomerular and alveolar basement membrane (ABM) is recognized to be the target antigen (Goodpasture antigen).[1] It is normally hidden in the α3, α4, α5 Protomer of Collagen IV of the GBM and ABM. It is turned into an autoantigen when an environmental factor like tobacco smoke or other hydrocarbon or infections damage the ABM, exposing the α-3 NC1 domain to the immune system,[2] evoking the anti-GBM (and anti-ABM) antibody formation.
Current literature suggests that anti-GBM antibody testing has high sensitivity and specificity for GBM disease.[3] O'Sullivan suggested sensitivity and specificity of 95–100% and specificity of 91–100% of anti-GBM assay by ELISA, as compared to sensitivity of 95.6% and specificity of 99.6% by chemiluminescence.[4]
Auto-antibody negative anti-GBM disease is increasingly being recognized. We currently test for anti-GBM antibody by fluoro-enzymatic assay in our immunology laboratory. The current serum was also tested in two other laboratories interstate (fluoro-enzymatic assay at one laboratory and by ELISA at the other) and reported negative for anti-GBM antibody, reassuring us that the negative anti-GBM antibody in our patient was the true result. Importantly, the patients current serum sample gave negative result on the same platform (ELISA) that had previously given positive results at the time of initial presentation, and therefore our opinion is that this patient indeed did not have anti-GBM antibody in the blood at the time of current testing, despite clear evidence of anti-GBM antibody binding in the glomerulus. Therefore, in the correct clinical context physicians should retain a high degree of diagnostic suspicion for anti-GBM disease, even in the absence of a positive anti-GBM assay, and renal biopsy remains the gold standard for diagnosis of Goodpasture's syndrome.
Antibody negative anti-GBM disease is being recognized increasingly, which may be accounted by changes in the antigenic target or due to variation in the antibody epitope. It may be due to antibodies against the α5 NC1 instead of the α3 NC1.[5] In other cases of seronegative anti-GBM disease, the antibody epitopes may be different, that is, IgG4 antibody instead of the usual IgG1 anti GBM antibody.[6] Antonelou et al. described a case of anti-GBM disease mediated by IgA anti-GBM antibody against collagenous domain of α1 Col (IV).[7] It is also possible that anti-GBM antibody in the blood may all become deposited in the kidney with time.
Lung hemorrhage with normal histology of kidney with linear anti-GBM staining has rarely been described in the literature previously.[89] A recent report from the Mayo clinic had described 20 patients with anti-GBM disease with indolent renal involvement, with negative serum anti-GBM antibody and no lung involvement.[10]
Recurrence of disease is more common in ANCA-associated vasculitis and in ''Double Positive'' patients who show both ANCA and anti-GBM positivity. In the largest study reported so far, double positive patients shared characteristics of ANCA-associated vasculitis like older age distribution and longer duration of symptoms before diagnosis, along with features of anti-GBM disease, that is, more severe renal disease and higher frequency of lung hemorrhage at presentation. However, overall patient survival was similar in both groups.[11] Our patient had negative ANCA, ANA, and normal complement levels at all times. All patients with anti-GBM disease should be strongly encouraged to quit smoking, given the strong association between cigarette smoking and pulmonary anti-GBM disease.[12]
This is an instructive case of a patient with anti-GBM disease with positive serology and crescentic GN in the past, presenting a decade later with a recurrent anti-GBM disease with lung hemorrhage, negative serology for anti-GBM Abs, an indolent renal involvement with normal renal function, and normal histology with linear IgG staining on IF on renal biopsy responded well to treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem. 1988;263:13374-80.
- [Google Scholar]
- Molecular architecture of the Goodpasture autoantigen in Anti-GBM nephritis. N Engl J Med. 2010;363:343-54.
- [Google Scholar]
- Goodpasture's disease in the absence of circulating anti-glomerular antibodies as detected by standard techniques. Am J Kidney Dis. 2002;39:1162-7.
- [Google Scholar]
- Goodpasture's syndrome: An example of an autoimmune process in the clinical and diagnostic setting. Australian J Med Sci. 2014;35:74-86.
- [Google Scholar]
- Circulating anti–glomerular basement membrane antibodies with predominance of subclass IgG4 and false-negative immunoassay test results in anti–glomerular basement membrane disease. Am J Kidney Dis. 2014;63:289-93.
- [Google Scholar]
- Binding truths: Atypical anti glomerular basement membrane disease mediated by IgA anti glomerular basement membrane antibodies targeting the α1 chain of Type IV collagen. Kidney Int Rep. 2019;4:163-7.
- [Google Scholar]
- Linear anti-glomerular basement membrane IgG but no glomerular disease: Goodpasture's syndrome restricted to the lung. Nephrol Dial Transplant. 2007;22:1233-5.
- [Google Scholar]
- The clinicopathologic characteristics and outcome of atypical anti-glomerular basement membrane nephritis. Kidney Int. 2016;89:897-908.
- [Google Scholar]
- Patients double-seropositive for ANCA and anti-GBM have varied renal survival, frequency of relapse, and outcomes compared to a single-seropositive patients. Kidney Int. 2017;92:693-702.
- [Google Scholar]
- Groupe d'Etudes et de Recherche sur les Maladies Orphelines Pulmonaires (GERMOP); Swiss Group for Interstitial and Orphan Lung Diseases (SIOLD). Alevolar hemorrhage in anti-glomerular basement membrane antibody disease: A series of 28 cases. Medicine (Baltimore). 2007;86:181-93.
- [Google Scholar]







