Translate this page into:
Fenofibrate Prevents nicotine-induced Acute Kidney Injury: Possible Involvement of Endothelial Nitric Oxide Synthase
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The present study investigated the possible effect of fenofibrate (peroxisome proliferator-activated receptors-α agonist) in nicotine-induced acute kidney injury (AKI) in rats.
Materials and Methods:
Nicotine (2 mg/kg/day, intraperitoneally) was administered for 4 weeks to induce AKI in rats. Lipid profile and renal oxidative stress were measured and expression of mRNA for eNOS was assessed using reverse transcription-polymerase chain reaction along with serum and renal tissue nitrite levels. Serum creatinine, blood urea nitrogen and microproteinuria were estimated along with the kidney histology, as markers of kidney function. Treatment with fenofibrate (30 mg/kg per oral, 4 weeks) was initiated 3 days before the administration of nicotine and continued for 4 weeks from the day of administration of nicotine.
Results:
Nicotine administered rats developed apparent AKI confirmed by elevated markers of kidney function and noticeable glomerulosclerosis and tubular cell degeneration. Nicotine decreases the expression of mRNA for eNOS, along with serum and renal tissue nitrite levels. In addition, nicotine showed significantly lipid alteration beside decrease oxidative stress, assessed in terms of increase in serum thiobarbituric acid reactive substance and a marked decrease in tissue reduced glutathione. However, fenofibrate significantly prevented the development of nicotine-AKI by reducing serum creatinine, BUN, and urinary protein, normalizing the lipid profile, reducing renal oxidative stress, increases the eNOS expression and concentration of serum and renal nitrate levels.
Conclusion:
Fenofibrate attenuates nicotine-induced AKI, via its antihyperlipidemic and antioxidant property. Moreover, fenofibrate induced upregulation of eNOS expression additionally play key roles in the improvement of nicotine-induced AKI could be the future alternative.
Keywords
Acute kidney injury
fenofibrate
nephroprotective
nicotine
Introduction
Cigarette smoking mediated nicotine exposure is considered to be a major risk factor involved in the induction and progression of reno-cardiovascular complications. Predominantly, smoking has been repeatedly confirmed as an independent risk factor for the development and progression of kidney disease in patients.[1]
The concealment information about the nicotine mediated kidney functional and structural abnormalities is by two major determinates; hyperliepedemia and high-grade oxidative stress.[23] Vascular endothelial dysfunction (VED) of microvessels results in reduced activation of endothelial nitric oxide synthase (eNOS), reduced generation and bioavailability of nitric oxide (NO) and increased production of reactive oxygen species (ROS).[45] VED, which is one of the important among the earliest stages has been associated in the pathogenesis of hypertension, coronary artery diseases and nephropathy.[67] A correlation between VED and kidney injury has been demonstrated in various studies.[89] VED has been shown to be involved in the induction and progress of nephropathy by inducing nodular glomerulosclerosis followed by glomerular basement membrane thickness and mesangial expansion.[910]
Evidently, it has been repeatedly proven that exposure of nicotine plays a key role in inducing VED.[478] This contention is supported by the fact that the nicotine-induced VED is due to downregulation of eNOS, hyperlipedemia and increased oxidative stress.[1112] In addition, nicotine is also noted to induce acute kidney injury (AKI) by accelerating glomerulosclerosis and provokes reno-vascular pathogenesis.[1314] Together, nicotine is forcefully inducing VED fallowed by the kidney injury by downregulating eNOS through hyperlipedemia and oxidative stress. However, no promising therapeutic option is available yet to attenuate nicotine mediated AKI and other vascular pathogenesis.
Fenofibrate is well known to produce antihyperlipidemic action by activation of peroxisome proliferator activated receptor-α (PPAR-α).[1015] It has been also noted that the fenofibarte upregulates the expression of eNOS, thus has been reported to ameliorate nicotine-induced endothelial dysfunction by reducing hyperlipidemia and oxidative stress.[1216] In addition, in our laboratory, we have shown that treatment with fenofibrate and concurrent administration of benfotiamine, prevented the development of diabetic nephropathy indicating fenofibrate-mediated renoprotection.[10] These studies certainly suggest a vascular and reno-protective potential of fenofibrate. However, the effect of fenofibrate in nicotine-induced AKI is not yet known. Thus, the growing evidence says that the use of fenofibrate attenuates nicotine-mediated VED-associated hyperlipidemia and oxidative stress.
From the above discussion, the fenofibrate can be considered to have pleiotropic actions and might have a potential to attenuate the renal functional and structural abnormalities. Therefore, the present study was aimed to explore the effect of fenofibrate as a possible therapeutic strategy to prevent nicotine-induced AKI.
Materials and Methods
Drugs and chemicals
The fenofibrate was obtained from Ranbaxy Laboratory Ltd., Gurgaon, India. 1,133 tetra methoxypropane and carboxymethyl cellulose were purchased from V. K. Chemicals and Instruments, Ambala, India. Nicotine was obtained from Nicosulf India Pvt. Ltd., Dakor, Gujarat, India. All other chemicals and kits used in the present study were of analytical grade.
Experimental animals
Age-matched young Wistar rats weighing about 200-250 g were housed in a room maintained at approximately 24 ± 1°C temperature and humidity of 55 ± 5% with 12-hour light/dark cycle. Free access to food (standard chow from Ashirwad Industries, India) and water was allowed. The rats were acclimatized for at least 3-4 days before the initiation of the experiment and were observed for any sign of disease. The rats were maintained under proper conditions till the termination of the experiment. Approval for all experiments was obtained from the institutional animal ethical committee (IAEC) and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Animal model and drug treatment
Nicotine (2 mg/kg/day, intraperitoneally [i.p.]) was administered for 4 weeks to induce AKI in rats. At the end of the study (4 weeks), the rats were sacrificed by cervical dislocation. Fenofibrate was administered 3 days before the administration of nicotine, and it was continued for 4 weeks from the day of administration of nicotine. All the parameters were assessed at the end of 4 weeks in normal and rats administered nicotine with or without drug treatments.
Experimental protocol
Three groups were employed in the present study and each group comprised of 8 animals. The fenofibrate was dissolved in 1% w/v of carboxy methylcellulose (CMC). Group I (normal control) rats were maintained on standard food and water and no treatment was given, group II (nicotine control) rats were administered nicotine (2 mg/kg/day, i.p., 4 weeks), and group III (fenofibrate-treated nicotine group) rats were treated with fenofibrate (30 mg/kg/day, per oral [p.o.]), the treatment was initiated 3 days before the administration of nicotine and continued for 4 weeks from the day of administration of nicotine.
Estimation of serum and renal tissue nitrite levels
Nitrite levels in serum and tissue were estimated as described earlier. The total nitrite content was expressed as nmoles per mg of protein.[17]
Estimation of mRNA expression for eNOS
The mRNA expression of renal aorta eNOS was performed as described earlier,[12] by RT-PCR technique and semiquantified using Bio1D software in gel documentation (Vilber Loumart, France). The primer sequence used was as follows. eNOS: Sense, 5′-CAC CCT CAG GTT CTG TGT GTT-3′; antisense, 5′-GTA GCC TGG AAC ATC TTC CGT-3′; β-actin: Sense, 5′-TGC TGT CCC TGT ATG CCT CT-3′; antisense, 5′- AGG TCT TTA CGG ATG TCAACG -3′.
Assessment of lipid profile
At the end of the experimental protocol, the blood samples were collected, and serum was separated after allowing to clot followed by centrifugation. The serum samples were frozen until analyzing the biochemical parameters. The serum total cholesterol and HDL cholesterol were estimated by cholesterol oxidase peroxidase (CHOD-POD) method,[17] using the commercially available kit. The serum triglyceride was estimated by glycerophosphate oxidase peroxidase (GPO-POD) method using the commercially available kit (Kamineni Life Sciences Pvt. Ltd., Hyderabad, India).
Assessment of oxidative stress
Estimation of serum thiobarbituric acid reactive substances (TBARS)
The serum concentration of TBARS was estimated spectrophotometrically to assess oxidative stress. A standard graph using 1, 1, 3, 3 tetramethoxypropane (1–50 μM) was plotted to calculate the concentration of TBARS.[47]
Estimation of renal glutathione (GSH)
Preparation of renal homogenate
The renal homogenate was mixed with 10% w/v trichloroacetic acid in 1:1 ratio and centrifuged at 4 C for 10 min at 5000 rpm. The supernatant (0.5 mL) was mixed with 2 mL of 0.3 M disodium hydrogen phosphate buffer (pH 8.4) and 0.4 mL of distilled water. Then, 0.25 mL of 0.001 M freshly prepared DTNB 5 50–dithiobis (2-nitrobenzoic acid) dissolved in 1% w/v sodium citrate] was added to the reaction mixture, and incubated for 10 min. The absorbance of the yellow colored complex was noted spectrophotometrically at 412 nm. A standard curve using the reduced form of glutathione was plotted to calculate the concentration of renal GSH. The renal GSH concentration was expressed as μM/g wet weight of renal tissue.[18]
Assessment of nicotine-induced AKI
The development of AKI in rats was confirmed 4 weeks after the administration of nicotine, and was assessed by measuring serum creatinine and blood urea nitrogen concentration using commercially available kits. In addition, histopathological studies were performed to assess nicotine-induced renal structural abnormalities. Markers of renal injury including serum level of creatinine estimated by alkaline picrate kinetic method, blood urea nitrogen was estimated by Berthelot method and urinary protein concentration was estimated by pyrogallol red method using commercially available kit (Span Diagnostics Ltd., India).[10]
Histopathological study
Nicotine-induced renal structural changes in glomeruli and tubules were assessed histologically. Briefly, the kidneys were excised and immediately immersed in 10% formalin. The kidney was dehydrated in graded concentrations of alcohol, immersed in xylene and then embedded in paraffin. From the paraffin blocks, sections of 5-μm thickness were made and stained with hematoxylin and eosin to assess pathological changes in glomeruli using light microscopy (200 ×).[10]
Statistical analysis
All values were expressed as mean ± S.E.M. The data for expression of mRNA for eNOS, nitrite level, serum TBARS, renal GSH, lipid profile, serum and tissue nitrates and creatinine, blood urea, urinary protein were statistically analyzed using one-way ANOVA followed by Dunnett test. The P value <0.05 was considered to be statistically significant.
Results
No mortality was observed in the animals within the 4 weeks of nicotine administration.
Effect of pharmacological interventions on serum and renal tissue nitrate level
The serum and renal nitrate level were noted to be reduced in nicotine administered rats when compared with normal rats. However, treatment with fenofibrate significantly attenuated nicotine-induced decrease in serum and renal level of nitrate. Fenofibrate produced marked restoration of decreased serum and renal nitrite level in rats administered nicotine [Table 1].
| Assessment | Normal Control | Nicotine | Fenofibrate treated Group |
|---|---|---|---|
| Serum cholesterol (mg/dl) | 70.12±6.6 | 174.25±9.1a | 98.4±3.01b |
| Triglycerides (mg/dl) | 80.2±6.6 | 145±10.1a | 95.8±7.2b |
| High density lipoprotein (mg/dl) | 38.6±04 | 30.1±0.8a | 36±0.7b |
| Serum TBAR (μM) | 3.5±0.3 | 8.3±0.26a | 6.75±0.56b |
| Renal GSH (μM/g wet weight of renal tissue) | 0.75±0.07 | 0.25±0.02a | 0.52±0.02b |
| Serum nitrite/nitrate (μM) | 13±0.29 | 7±0.5a | 11.3±0.3b |
| Tissue nitrite/nitrate (μM/mg of protein) | 17±0.4 | 6±0.3a | 13±0 0.35b |
All values are represented as mean±SD. aP<0.05 vs. normal control. bP<0.05 vs nicotine control
Effect of pharmacological interventions on expression of mRNA for eNOS
Nicotine-induced AKI reduced expression expression ratio of eNOS mRNA. The fenofibrate markedly prevented the nicotine induced decrease in expression ratio of eNOS indicating increased NO production in the kidney [Figure 1].
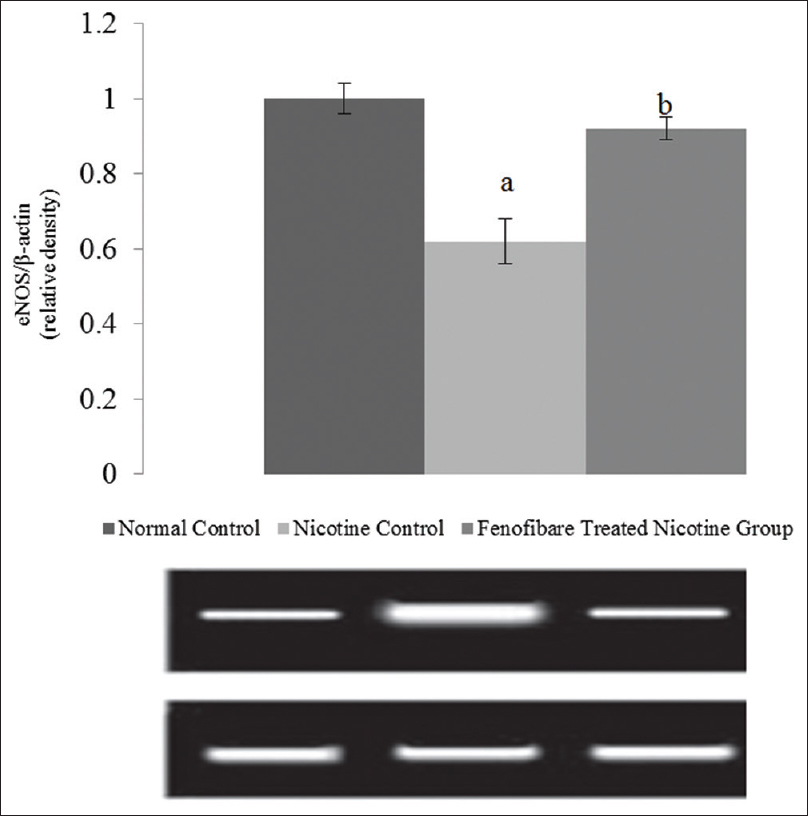
- Effect of fenofibrate on eNOS expression. All values are represented as mean ± S.D. a = p < 0.05 vs Normal Control; b = p < 0.05 vs Nicotine Control
Effect of pharmacological interventions on serum lipid concentration
The significant increase in serum concentration of total cholesterol and triglycerides and decrease in HDL were noted in rats with nicotine mediated AKI when compared with normal rats. However, treatment with fenofibrate markedly reduced the nicotine-induced alterations in serum lipids [Table 1].
Effect of pharmacological interventions on serum TBARS and renal glutathione
Marked increase in serum TBARS and decrease in renal glutathione was noted in nicotine-administered rats. However, treatment with fenofibrate significantly attenuated nicotine-induced increase in serum TBARS concentration and decrease in renal glutathione [Table 1].
Effect of pharmacological interventions on serum creatinine, blood urea, and proteinuria
The serum creatinine, blood urea nitrogen and proteinuria levels were increased in nicotine-administered rats as compared to normal rats. However, treatment with fenofibrate reduced the nicotine-induced high serum concentrations of creatinine, blood urea, and elevated urinary protein [Figures 2-4].
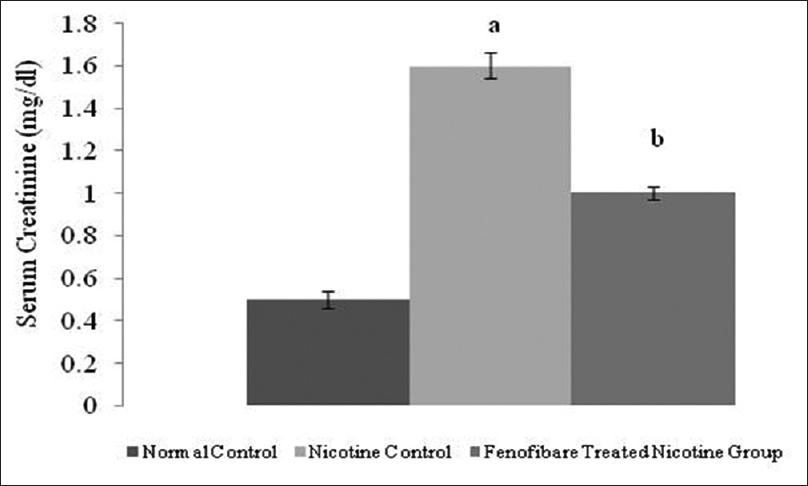
- Effect of fenofibrate on serum creatinine. All values are represented as mean ± S.D. a = p < 0.05 vs Normal Control; b = p < 0.05 vs Nicotine Control
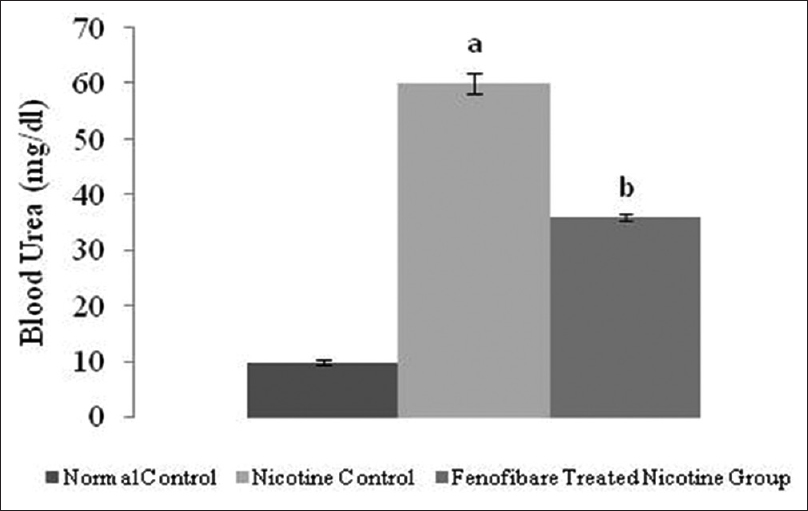
- Effect of fenofibrate on blood urea nitrogen. All values are represented as mean ± S.D. a = p < 0.05 vs Normal Control; b = p < 0.05 vs Nicotine Control
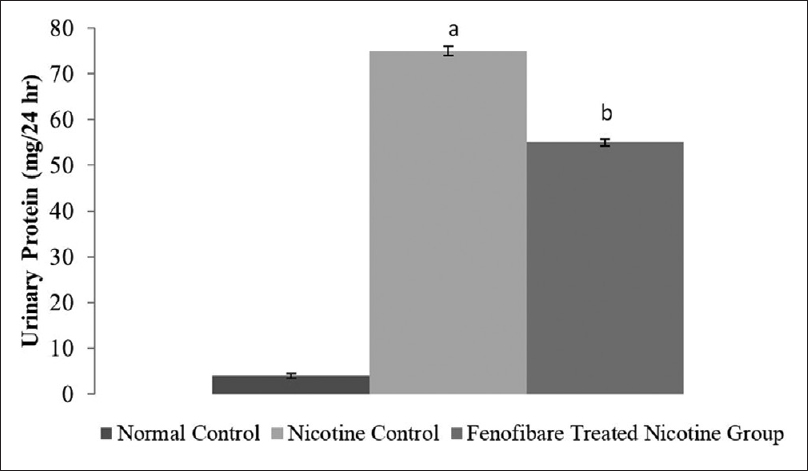
- Effect of fenofibrate on urinary Protein. All values are represented as mean ± S.D. a = p < 0.05 vs Normal Control; b = p < 0.05 vs Nicotine Control
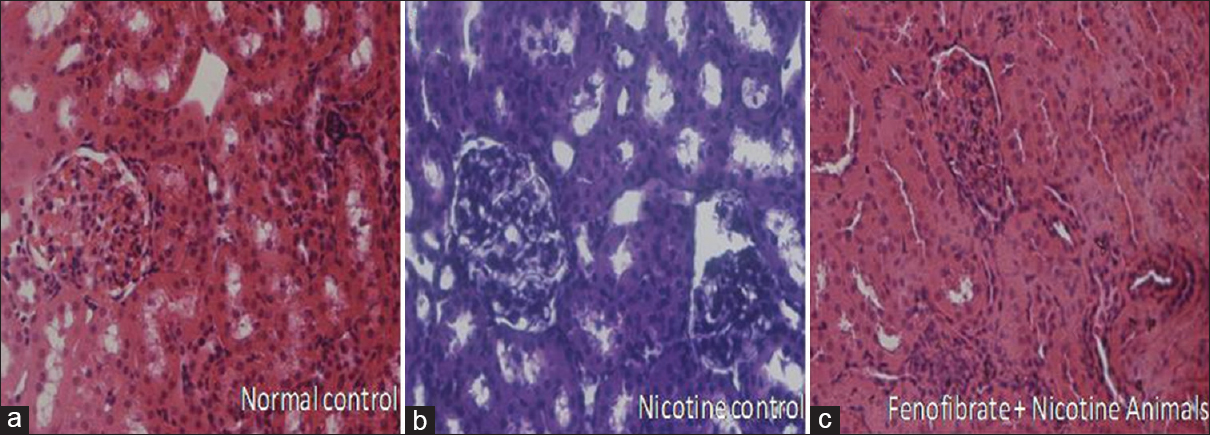
- Histopathological changes associated with nicotine and treatment groups (a: Normal Control, b: Nicotine Control, c: Fenofibrate treated nicotine group)
Effect of pharmacological interventions on histopathological study on kidney
The significant renal structural pathological abnormalities in the glomerulus and tubules were observed in nicotine-administered rats. The pathological changes in the glomeruli such as reduced capillary size and extracellular mesangial expansion were noted in nicotine administered rats after 4 weeks when compared with normal rats. Treatment with fenofibrate reduced the pathological changes in glomeruli by improving the glomerular capillary size and reducing the mesangial expansion [Figure 5].
Discussion
The increasing prevalence of AKI calls for stronger measures to halt the progression of kidney injury, and smoking cessation maybe a valuable tool in this direction. Smoking contributes to renal disease progression by eliciting microvascular injury and accelerates global glomerulosclerosis.[13] In addition, it has been noted that smoking reportedly exerts deleterious effects on renal function and structure.[1419] Nicotine exposure via chronic cigarette smoking is an emerging cause that accelerates the microvascular complications. Recent evidence suggests that nicotine forcefully induces the kidney injury in rats.[1920] It is very difficult to understand smoking-mediated microvascular wiggles because nicotine has intricate mechanisms. In addition, nicotine is responsible for eNOS downregulation and induces VED in rats, which is a key step in the pathogenesis of AKI.[91221] Moreover, nicotine also noted to worsen the nephropathy by activation of hyperlipidemia and high-grade oxidative stress.[2122]
Fenofibrate, an activator of PPAR-α, is a well-known therapeutic drug for lipid management.[10] PPAR-α activation results in the activation of lipoprotein lipase and increases lipolysis and reduced triglyceride levels.[23] It is interesting to note that nicotine alters the lipid profile by increasing the level of LDL and total cholesterol and consequently decreases the level of HDL.[24] Nicotine causes impairment of lipoprotein lipase, an enzyme involved in the hydrolysis and clearance of triglycerides from the circulation.[2526] Thus, nicotine-induced kidney damage is often associated with hypercholesterolemia and hypertriglyceridemia. This contention is supported by the results obtained in the present study that the total cholesterol and triglycerides levels were noted to be markedly reduced and HDL level gets increased after fenofibrate treatment in nicotine administered rats.
The last decade has seen several significant studies, both on animal models, as well as human subjects, to strongly suggest the role of cigarette smoking in the progression of renal failure. Nicotine administered kidneys are more susceptible to oxidative damage because of the induction of deficiency in antioxidant defense enzymes. Similar kind of reduction of antioxidant level and increase in oxidative stress has been observed in previous studies where nicotine in renal tubular cells produced oxidative stress and reduced the level of SOD (superoxide dismutase) along with a reduction in renal cell viability.[2728] Nicotine frequently demonstrated oxidative stress and risk factor for vascular complications.[1214] Moreover, nicotine induces oxidative stress by generating ROS via activation of NADPH oxidase, assessed in terms of increase in serum TBARS and superoxide anion generation and increase in expression of mRNA for p22phox in other studies.[1222] For instance, nicotine has a potent effect on promoting the development of oxidative damage in renal tissue. This is further supported by the fact that in the present study the nicotine administered rats showed high-grade oxidative stress by increasing serum and tissue TBARS. Additionally, a marked decrease in renal GSH noted in nicotinic rats as compared to normal rats indicate nicotine-induced oxidative stress, which is reversed by the treatment with fenofibrate.
In previous observations, vascular endothelium plays an important role in the pathogenesis of kidney injury.[910] Few clinically and preclinical studies have documented that the nicotine plays a key role in mediating endothelial dysfunctions by decreasing the generation and bioavailability of NO and downregulating the expression of eNOS, which is the first step in the pathogenesis of AKI.[122930] Fenofibrate has been noted to improve the function of endothelium mediated renal damage by increasing the bioavailability of NO and reducing the oxidative stress.[101112131415161718192021223132] The vascular protecting potential of fenofibrate in preventing the development of vascular endothelial dysfunction and kidney injury may be attributed to its antihyperlipidemic property and/or its pleiotropic actions such as activation of eNOS and generation of NO and the consequent reduction in oxidative stress to improve the integrity and function of the endothelium and renal functions in nicotine administered rats. Nicotine decreases in expression of mRNA for eNOS and its treatment with fenofibrate prevented the nicotine induced decrease in eNOS. These results support the fenofibrate-mediated vascular and reno-protective effects obtained in the present study.
AKI is defined as a multifactorial syndrome, characterized by an abrupt loss of renal function which results in the accumulation of metabolic waste and toxins, such as serum creatinine and blood urea nitrogen, and/or decreased urine output.[333435] The increase in serum creatinine and blood urea and pathological changes in glomeruli have been documented to be an index of AKI.[1033] We observed in the present study that nicotine administered rats exhibited a marked elevation in serum creatinine. These results suggest the induction of AKI in nicotine-administered rats. The morphological changes in the kidney are considered as direct evidence for the confirmation of AKI. In the present study, histopathological analysis revealed renal structural abnormalities that occurred in glomerulus and tubules, with degeneration in the glomerular wall and mild hypertrophy in glomerulus were noted in nicotine-administered rats. These results suggest the development of nicotine-induced AKI. It is noteworthy that fenofibrate plays pleiotropic actions in the prevention of kidney injury. In support of this proposal, the fenofibrate mediated multifactorial potential is explored. Our study has demonstrated for the first time that pleiotropic actions of fenofibrate in the nicotine-induced AKI by reducing hyperlipidemia, oxidative stress subsequently upregulated the expression of eNOS and have played a key role in the protection of renal function. Pondering over the above discussion, fenofibrate might have a therapeutic potential to prevent nicotine induced renal functional and structural abnormalities. Besides cardiopretection, fenofibrate could play key roles in the prevention of AKI.
Conclusion
Fenofibrate have afforded protection against nicotine-induced kidney damage, therefore, these agents warrant further investigations in the animal models of kidney damage and can be utilized in the therapeutic management of nicotine induced kidney damage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
I wish to express my gratitude to my beloved father Late. Mr. Arvind S. Chakkarwar, for his inspiration to do research and valuable suggestions. I also express my thankfulness to my guide Dr. Pravin Kawtikwar, Principal SNIOP, Pusad, for his praiseworthy suggestion and constant support for this study. The proofreading support from the Prime Editors, the scientific editing company (www.primeeditors.in) Aurangabad, is highly appreciable.
References
- Independent and combined effect of bilirubin and smoking on the progression of chronic kidney disease. Clin Epidemiol. 2018;10:121-32.
- [Google Scholar]
- Investigating the role of novel bioactive compound from ficus virens ait on cigarette smoke induced oxidative stress and hyperlipidemia in rats. Iran J Pharm Res. 2017;16:1089-1103.
- [Google Scholar]
- Smoking in diabetic nephropathy: Sparks in the fuel tank? World J Diabetes. 2012;3:186-95.
- [Google Scholar]
- Nitric oxide donors as potential drugs for the treatment of vascular diseases due to endothelium dysfunction. Curr Pharm Des. 2020;26:3748-59.
- [Google Scholar]
- Chronic cigarette smoke exposure triggers a vicious cycle of leukocyte and endothelial-mediated oxidant stress that results in vascular dysfunction. Am J Physiol Heart Circ Physiol. 2020;319:H51-65.
- [Google Scholar]
- Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide. 2018;78:113-20.
- [Google Scholar]
- Endothelium-dependent and -independent vascular function in advanced chronic kidney disease. Clin J Am Soc Nephrol. 2017;12:1588-94.
- [Google Scholar]
- Diabetic nephropathy and endothelial dysfunction: Current and future therapies, and emerging of vascular imaging for preclinical renal-kinetic study. Life Sci. 2016;166:121-30.
- [Google Scholar]
- Vascular endothelial dysfunction: A tug of war in diabetic nephropathy? Biomed Pharmacother. 2009;63:171-9.
- [Google Scholar]
- Ameliorative effect of combination of benfotiamine and fenofibrate in diabetes-induced vascular endothelial dysfunction and nephropathy in the rat. Mol Cell Biochem. 2009;320:149-62.
- [Google Scholar]
- Roselle supplementation prevents nicotine-induced vascular endothelial dysfunction and remodelling in rats. Appl Physiol Nutr Metab. 2017;42:765-72.
- [Google Scholar]
- Fenofibrate attenuates nicotine-induced vascular endothelial dysfunction in the rat. Vascul Pharmacol. 2011;55:163-8.
- [Google Scholar]
- Resveratrol supplementation protects against nicotine-induced kidney injury. Int J Environ Res Public Health. 2019;16:4445.
- [Google Scholar]
- Effect of harmine on nicotine-induced kidney dysfunction in male mice. Int J Prev Med. 2019;10:97.
- [Google Scholar]
- Effect of fenofibrate on serum nitric oxide levels in patients with hypertriglyceridemia. Adv Clin Exp Med. 2019;28:931-6.
- [Google Scholar]
- Fenofibrate improves vascular endothelial function in diabetic mice. Biomed Pharmacother. 2019;112:108722.
- [Google Scholar]
- Spectrophotometric determination of serum nitrite and nitrate by copper–cadmium alloy. Anal Biochem. 2002;306:79-82.
- [Google Scholar]
- Nephroprotective effect of catechin on gentamicin-induced experimental nephrotoxicity. Clin Exp Nephrol. 2015;19:178-84.
- [Google Scholar]
- Nicotine worsens the severity of nephropathy in diabetic mice: Implications for the progression of kidney disease in smokers. Am J Physiol Renal Physiol. 2010;299:F732-9.
- [Google Scholar]
- Renoprotective effect of Spirulina platensis extract against nicotine-induced oxidative stress-mediated inflammation in rats. Phytomedicine. 2018;49:106-10.
- [Google Scholar]
- Contribution of cathepsin B-dependent Nlrp3 inflammasome activation to nicotine-induced endothelial barrier dysfunction. Eur J Pharmacol. 2019;865:172795.
- [Google Scholar]
- Nephroprotective and antioxidant effect of green tea (Camellia sinensis) against nicotine-induced nephrotoxicity in rats and characterization of its bioactive compounds by HPLC-DAD. Appl Physiol Nutr Metab. 2019;44:1134-40.
- [Google Scholar]
- Endothelial to mesenchymal transition contributes to nicotine-induced atherosclerosis. Theranostics. 2020;10:5276-89.
- [Google Scholar]
- Alterations of lipolysis and lipoprotein lipase in chronically nicotine-treated rats. Am J Physiol. 1996;270:E215-23.
- [Google Scholar]
- Fenofibrate, a PPARalpha agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int. 2011;79:871-82.
- [Google Scholar]
- Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension. 2012;60:1517-23.
- [Google Scholar]
- Environmental tobacco smoke exposure during pregnancy has limited effect on infant birthweight and umbilical vein endothelial nitric oxide synthase. Acta Obstet Gynecol Scand. 2018;97:1309-16.
- [Google Scholar]
- Oral nicotine aggravates endothelial dysfunction and vascular inflammation in diet-induced obese rats: Role of macrophage TNFα. PLoS One. 2017;12:e0188439.
- [Google Scholar]
- High-fat diet-induced renal cell apoptosis and oxidative stress in spontaneously hypertensive rat are ameliorated by fenofibrate through the PPARα-FoxO3a-PGC-1α pathway. Nephrol Dial Transplant. 2012;27:2213-25.
- [Google Scholar]
- PPAR-α agonist fenofibrate ameliorates oxidative stress in testicular tissue of diabetic rats. Crit Rev Eukaryot Gene Expr. 2020;30:93-100.
- [Google Scholar]
- Diagnostic performance of serum blood urea nitrogen to creatinine ratio for distinguishing prerenal from intrinsic acute kidney injury in the emergency department. BMC Nephrol. 2017;18:173.
- [Google Scholar]
- Resveratrol attenuates nicotine-mediated oxidative injury by inducing manganese superoxide dismutase in renal proximal tubule cells. In Vivo. 2017;31:551-5.
- [Google Scholar]







