Translate this page into:
Clinical Profile and Outcome of Hemodialysis Patients with SARS COV2 Infection in a Tertiary Care Centre in Mumbai, India
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
CKD5D is a high risk subgroup with high comorbidity burden, need for frequent visits to dialysis centre and a compromised immune system. The effect of SARS COV2 virus on this population is not well known.
Methods:
This prospective study enrolled, all CKD5D with COVID 19 infection, admitted to our hospital, from 23rd April to 30th June 2020 & whose outcome as discharge/mortality was known. Their clinical profile, investigations, treatment and outcome in terms of mortality or discharge after clearing infection was noted and analysed.
Results:
Total 203 dialysis patients with COVID 19 were referred to our institute. Of these total, 131 were analysed. Median age was 50 years (19-80 years) with 57% were males. Hypertension (76%) was the commonest comorbidity followed by diabetes (29%) and coronary artery disease (22%). Dyspnoea, fever and cough were present in 50%, 40%, and 33% patients respectively. 26% were asymptomatic. None had dialyser clotting. Mortality was 20.6%. Time to turn RT PCR negative was 14 days (3-40 days). Comparing deceased vs survivors: Age [56 vs 49 yrs], diabetes [56% vs 22%], duration of symptoms at admission [5 vs 4 days], dyspnea [85% vs 40%] and encephalopathy [30% vs 1%] at admission, bilateral opacities on Chest X ray [93% vs 20%] and high leucocyte count [11,059 ± 5,929 vs 7,022 ± 2,935/cmm] were statistically significant variables associated with mortality.
Conclusion:
Asymptomatic group was 26% of the total CKD5D with COVID 19 infection population analysed. Mortality was 20.61%. Higher age, later presentation to hospital, diabetes, dyspnoea, & encephalopathy at presentation, bilateral opacities on Chest X- Ray & higher leukocyte counts were significantly associated with mortality.
Keywords
COVID 19
dialysis
end stage renal disease
India
Introduction
A novel virus SARS COV 2 surfaced in China December 2019 and has spread across the globe affecting millions of patients and causing havoc to the normal order of the world. WHO declared COVID 19 as a pandemic in March 2020. India saw its first case on 30th January 2020 in Kerala.[1] Ever since the disease has spread all over the country with Maharashtra having more than 4,00,000 cases as of 29th July 2020 and Mumbai emerging as an infection hotspot in India.[2] To tackle this unprecedented situation, in Mumbai, two multi-speciality hospitals were converted into Dedicated COVID Hospitals to care for COVID 19 patients. Ours, T.N. Medical College & BYL Nair Ch Hospital was one of these.
The current data suggests that this virus although affecting all age groups, causes greater morbidity and mortality in patients with comorbidities like hypertension, diabetes, obesity, lung disease and heart disease.[3] The presentation can range from asymptomatic infection to bilateral pneumonia with severe acute respiratory syndrome. It can affect various organs like cardiovascular, gastrointestinal tract, nervous system, blood and kidneys.[4]
Chronic Kidney Disease (CKD) patients have a high proportion of co-morbidities and are immunocompromised, making them more susceptible to infections.[5] The first COVID 19 death reported in USA was an ESRD patient on Haemodialysis.[6] Home isolation is not an option as patients on maintenance haemodialysis (MHD) have to visit their dialysis centre two to three times a week. Studies have shown mortality rate ranging from 16% to 30.5%.[78] Also, analysis of CKD5D patients on MHD of Wuhan revealed that upto 21.4% patients can be asymptomatic carriers and haemodialysis centres can be high risk settings for spread of infection.[9]
In this study, we focus on hemodialysis patients with COVID19 infection with emphasis on epidemiology, including clinical features & outcome & its corelation to presenting features.
Methods
This was an prospective observational single centre study to assess clinical profile, and outcome of hemodialysis patients with SARS COV2 infection during pandemic in a tertiary care hospital in a Metro city, from 23rd April 2020 to 30th June 2020.Permission from Institutional Ethics Committee was obtained.
Inclusion Criteria
Patients with all the following were included.
All adult patients of CKD5D admitted in our hospital and dialysed at our centre
SARS COV2- RT PCR positive: two positive reports [one at admission & one 2 days later]
Known outcome at discharge
Written informed consent.
Exclusion Criteria
Non-dialysis CKD and SARS COV2- RT PCR positive
Acute kidney injury and acute on CKD
CKD initiated on dialysis during this admission
Age group <18 years.
Primary End Point
Outcome: Discharge vs Death.
Secondary End Points
Corelation of outcome with co morbidities & clinical presentation
Time taken to turn seronegative.
Their demographic data, co morbidities, investigations details including hematologic & biochemical reports, Radiography (Xray chest &/or CT Chest), SARS COV2 RT PCR reports, treatment details were noted. Clinical course in terms of inotrope requirement, need for oxygen therapy or any other organ dysfunction were recorded. Therapy evolved and changed with time & adopted as per availability, as expected in this new pandemic. They were followed for their entire course of hospital stay. Time required for turning RT PCR negative and final outcomes that is, discharge/mortality, were noted.
MOHFW guidelines & precautions for Haemodialysis during COVID pandemic were followed.[10] At the start of the pandemic, as our institute received large number of dialysis referrals, our resources were outnumbered. Few of these patients had missed 2 to 3 sessions of MHD due to fear of visiting hospitals/dialysis centers and lockdown. Hence to accommodate all patients, initially MHD sessions were done twice a week during the first two weeks of the study period. However subsequently, as more dialysis machines, portable RO were acquired and more dialysis technicians recruited, everyone received three, 4 hours dialysis sessions per week. Dialysers were not reused. Regular Heparin was used during dialysis [40 units/kg as bolus & 12 units/kg as maintenance]. Systemic Heparin was used, 5000 units twice daily, in all those needing oxygen. Later group did not receive any heparin during MHD. Complications, during MHD, were noted, if any.
Discharge policy
Ours is a Public Health Institute with main dialysis load of acute kidney injury (AKI) and Acute on CKD. All MHD patients were referrals as they were either symptomatic needing admission or could not be accommodated at their parent centres due to lack of COVID specific shift/isolation facility. Most patients had missed 2 to 3 sessions prior to admission. To handle this load and ensure that everyone received adequate dialysis, following policy was made. They were discharged if they were asymptomatic for at least 3 days, along with 2 consecutive SARS COV2 RT PCR negative reports and with creatinine less than 10 mg % and serum potassium less than 5.5 meq/L.
Statistical analysis
The descriptive statistics is presented in terms of frequency (counts) and percentages (%) in different categories for qualitative variables and for quantitative variables as range (minimum, maximum) and as means and standard deviations/median & standard error of mean. The statistical significance of qualitative/categorical variables was determined by Chi Square test. Subsequently, logistic regression was used to confirm the effects of qualitative/categorical variables and test the effects of continuous variables. The software used for logistic regression was SAS Version 9.4.
Results
A total of 4324 patients with SARS COV2 infection were admitted in the hospital, of which 203 patients were dialysed from 23rd April to 30th June 2020 at our center [Table 1]. Of these 21 patients were AKI, 16 were acute on CKD, 7 were CKD5 who were initiated on dialysis and they were excluded from the study. 159 were CKD5D already on maintenance haemodialysis out of which 28 were still admitted at time of writing this manuscript and were also excluded from the study. Outcomes were available for 131 MHD patients. Of the 131 patients, a total of 104 patients turned RT PCR negative and were discharged (Survivors) and 27 patients died (Deceased). Median time from onset of symptoms to discharge was 21 days (8 to 43 days) while from onset of symptoms to death was 12 days (range 4-41 days). The mortality of ESRD patients with COVID 19 was found to be 20.61%.
| All patients dialysed | ESRD on Maintenance Haemodialysis | |
|---|---|---|
| Total patients (n) | 203 [ESRD, AKI, AKI on CKD] | 159 |
| Still admitted/transferred (n) | 38 | 28 |
| Outcomes achieved (n) | 165 | 131 |
| Expired (n) | 47 | 27 |
| Mortality (%) | 28.48% | 20.61% |
ESRD: End Stage Renal Disease AKI: Acute Kidney Injury CKD: Chronic Kidney Disease
Clinical details, treatment and course of disease
The median age of the cohort was 50 years, with males being 57%. Other demographic details are shown in Table 2. At presentation, 34 (26%) patients were asymptomatic. Some were tested as part of post exposure testing protocol and some as part of routine screening protocols of certain dialysis centres. Haemoglobin, total leucocyte count (TLC), platelet counts, serum creatinine, serum sodium and potassium levels, when compared between the survivors and deceased, it were similar except for TLC, which was significantly higher in deceased. The Chest X-ray at admission showed bilateral opacities in 35%, unilateral opacities in 6%, pleural effusion in 5% and was normal in 53%. 93% patients in the deceased group had bilateral opacities on admission.
| ALL | SURVIVORS | DECEASED | P | |
|---|---|---|---|---|
| Total (n) | 131 | 104 | 27 | |
| Age in years (n) (median; range) | 50 (19-80) | 49 (19-76) | 56 (21-80) | 0.0047 |
| Male: n (%) | 75 (57%) | 58 (56%) | 17 (63%) | 0.5017 |
| Female: n (%) | 56 (43%) | 46 (44%) | 10 (37%) | |
| Co morbidities: n (%) | ||||
| Hypertension | 99 (76%) | 77 (74%) | 22 (81%) | 0.425 |
| DM | 38 (29%) | 23 (22%) | 15 (56%) | 0.001 |
| CAD | 29 (22%) | 20 (19%) | 9 (33%) | 0.1201 |
| Underlying Respiratory illness | 11 (8%) | 8 (77%) | 3 (11%) | 0.570 |
| HBV | 0 | 0 | 0 | |
| HIV | 0 | 0 | 0 | |
| HCV | 5 (4%) | 4 (4%) | 1 (4%) | |
| Hypothyroidism | 6 (5%) | 6 (6%) | 0 | |
| Renal Allograft Failure | 2 (1.5%) | 2 (2%) | 0 | |
| Dialysis Vintage in years (median; Range) | 2 (0.1-14) | 2 (0.1-14) | 2 (0.4-12) | 0.807 |
| Dialysis Access: n (%) | ||||
| Arterio-venous fistula | 98 (75%) | 76 (73%) | 22 (81%) | 0.373 |
| Dialysis Catheter | 33 (25%) | 28 (27%) | 5 (18.5%) | |
| Double Lumen catheter | 13 (10%) | 10 (10%) | 3 (11%) | |
| Tunnelled cuffed Catheter | 20 (15%) | 18 (17%) | 2 (7%) | |
| Frequency of dialysis [Before referral to us] | 0.757 | |||
| 3/WK: n (%) | 84 (64%) | 66 (63%) | 18 (67%) | |
| <3/WK: n (%) | 47 (36%) | 38 (36.5%) | 9 (33%) | |
| 1/WK | 1 (1%) | 1 (1%) | 0 | |
| 2/WK | 46 (35%) | 37 (35.5%) | 9 (33%) | |
| Clinical Presentation | ||||
| Asymptomatic n (%) | 34 (26%) | 34 (33%) | 0 | <0.001 |
| Symptomatic n (%) | 97 (74%) | 70 (67%) | 27 (100%) | |
| Duration in days (median; range) | 5 (1-12) | 4 (1-12) | 5 (2-10) | 0.003 |
| Symptoms at Admission n (%) | ||||
| Fever | 52 (40%) | 37 (35.5%) | 15 (55.5%) | 0.062 |
| Cough | 43 (33%) | 35 (34%) | 8 (30%) | 0.691 |
| Dyspnea | 65 (50%) | 42 (40%) | 23 (85%) | <0.001 |
| Gastrointestinal Symptoms | 19 (14.5%) | 16 (15%) | 3 (11%) | 0.576 |
| Encephalopathy | 9 (7%) | 1 (1%) | 8 (30%) | <0.001 |
| Fluid Overload (anasarca/edema) | 10 (7%) | 6 (6%) | 4 (15%) | 0.127 |
| Investigations (mean; SD) | ||||
| Haemoglobin (g%) | 8.78±1.77 | 8.81±1.76 | 8.65±1.87 | 0.671 |
| Total leucocyte count (/cmm) | 7,854±4,067 | 7,022±2,935 | 11,059±5,929 | <0.001 |
| Platelet count (/cmm) | 1,97,916±1,06,528 | 2,01,452±1,07,853 | 1,84,296±1,02,064 | 0.455 |
| Serum creatinine (mg%) | 11.28±6.47 | 11.59±7.03 | 10.07±3.42 | 0.285 |
| Serum sodium (mEq/L) | 137±4.54 | 137±4.21 | 137±5.71 | 0.964 |
| Serum potassium (mEq/L) | 5.0±0.96 | 4.99±0.95 | 5.05±1.01 | 0.753 |
| Chest X ray n (%) | ||||
| Normal | 70 (53%) | 69 (66%) | 1 (4%) | |
| Bilateral opacities | 46 (35%) | 21 (20%) | 25 (93%) | <0.001 |
| Unilateral opacities | 8 (6%) | 7 (7%) | 1 (4%) | 0.056 |
| Pleural Effusion | 7 (5%) | 7 (7%) | 0 | |
| Clinical course n (%) | ||||
| No oxygen requirement | 76 (58%) | 74 (71%) | 2 (7%) | <0.001 |
| Requiring oxygen | 55 (42%) | 30 (29%) | 25 (93%) | <0.001 |
| Requiring inotropes | 11 (8%) | 1 (1%) | 10 (37%) | <0.001 |
| Treatment n (%) | ||||
| Hydroxychloroquine | 65 (50%) | 51 (49%) | 14 (52%) | Not req |
| Azithromycin | 82 (63%) | 61 (59%) | 21 (78%) | Not req |
| Oseltamavir | 8 (6%) | 2 (2%) | 6 (22%) | Not req |
| Antibiotics | 75 (57%) | 54 (52%) | 21 (78%) | Not req |
| Ivermectin | 23 (17.5%) | 16 (15%) | 7 (26%) | Not req |
| Lopinavir | 14 (10.7%) | 9 (9%) | 5 (18.5%) | Not req |
| Steroid | 9 (7%) | 5 (5%) | 4 (15%) | Not req |
DM: Diabetes Mellitus CAD: Coronary Artery Disease SLE: Systemic Lupus Erythematosus HBV: Hepatitis B Virus HCV: Hepatitis C Virus HIV: Human Immunodeficiency Virus
Over the two months, treatment protocols changed with gathering evidence. Patients received hydroxychloroquine (50%), azithromycin (63%), oseltamivir (6%), ivermectin (17.5%) and antibiotics (57%). Steroids (7%) were given as methylprednisolone 0.5 to 1 mg/kg/day for 5 to 7 days to all who needed oxygen therapy. Other therapies included lopinavir in 10.7%. None of the patients included in this study received tocilizumab, remdesivir or favipravir. Heparin was used in all patients who needed oxygen therapy, 5000 units twice daily.
Outcome was assessed as discharge or death. Of total 131 patients, 104 were discharged & 27 expired. Median time from onset of symptoms to death was 12 days (range 4-41 days) while Median time from onset of symptoms to discharge was 21 days (range 8-43 days). During hospital stay, 76 (58%) patients did not develop ARDS and remained stable. ARDS developed in 55 (42%) patients. Eleven patients (8%) developed shock requiring inotrope support and SLED was provided to this group. Oxygen therapy was given via nasal prong, bag and mask ventilation, and mechanical ventilation. Twenty five of the 55 (45.5%) patients with ARDS eventually died, of whom 20 patients had ARDS on admission, 5 were stable on admission and developed ARDS during hospital stay. No clotting of dialysers was noted. AVF clotting was seen in 5% and AVF hematoma/bleeding seen in 5%.
RT PCR was done every 2nd to 3rd day in indoor patients and they were discharged if they had been asymptomatic for at least 3 days with 2 RT PCR negative reports. 104 of 131 patients were discharged. These patients remained RT PCR positive for a median of 14 (range 3-40) days from onset of symptoms. We considered day of detection as day of onset of symptom in asymptomatic patients.
Asymptomatic vs Symptomatic
Baseline characteristics of asymptomatic vs symptomatic [Table 3] group did not show statistical significant difference except for ARDS manifestation. ARDS was feature in 55% (n = 53) in symptomatic group vs only 6% (n = 2) of asymptomatic group. Complete recovery was noted in latter group. Asymptomatic group overall had good prognosis and a milder disease course.
| Asymptomatic (n=34) | Symptomatic (n=97) | P | |
|---|---|---|---|
| Age in years (median; range) | 48 (22-76) | 50 (19-80) | 0.275 |
| Male | 19 (56%) | 56 (58%) | 0.851 |
| Hypertension | 24 (70.5%) | 75 (77%) | 0.433 |
| DM | 9 (26.5%) | 29 (30%) | 0.705 |
| CAD | 9 (26.5%) | 20 (21%) | 0.480 |
| Respiratory illness | 0 | 11 (11%) | 0.004 |
| Dialysis Vintage | |||
| <2 years | 18 (53%) | 50 (51.5%) | 0.928 |
| >2 years | 16 (47%) | 47 (48.5%) | |
| Dialysis Access | 0.841 | ||
| AV Fistula | 25 (73.5%) | 73 (75%) | |
| Dialysis Catheter | 9 (26.5%) | 24 (25%) | |
| Frequency of Dialysis | 0.934 | ||
| <3/Wk | 12 (35%) | 35 (36%) | |
| >3/Wk | 22 (65%) | 62 (64%) | |
| Clinical course | |||
| ARDS | 2 (6%) | 53 (55%) | <0.001 |
| Days from SARS COV2 Detection to RT PCR Negative | 13.29±9.57 | 13.48±8.17 | 0.951 |
| Mortality (n) | 0 | 27 (28%) |
Factors associated with increased mortality
Patients who were discharged after negative RT PCR report (n = 104) were compared to deceased (n = 27) [Table 2]. Chi square test and logistic regression were used to determine if any factors had a significant effect on likelihood of death. We found that increasing age (Wald χ2(1) = 7.9911; P = 0.0047) and diabetes (wald χ2 (1) = 10.6723, P = 0.0011) had a significant effect on the likelihood of death. Specifically, participants were more likely to die when they had diabetes (39.47%) than when they didn't (12.90%). The likelihood of death with age more than 50 years, 60 yrs and 80 years was 19.28%, 28.96% and 54.31% respectively. Other comorbidities like hypertension, coronary heart disease or underlying respiratory illness or even dialysis associated factors were not found to be associated with mortality.
The average duration of symptoms at admission was slightly longer in deceased as compared to survivors (5 vs 4 days), and the difference was statistically significant. All [100%] diseased were symptomatic while only 67% of survivors were symptomatic [P < 0.001]. The logistic regression analysis showed that the likelihood of mortality was significantly higher when patients presented with, dyspnea and encephalopathy at presentation [Table 4]. Oxygen therapy was necessary in 93% of diseased & in only 29% of survivors [P < 0.001]. Difference in ionotropic support between deceased & survivor groups (37% vs 1%) was of statistical significance [P < 0.001] Fever and fluid overload showed a trend towards higher association with mortality; however the P value was not significant.
| Probability of death | P | ||
|---|---|---|---|
| Diabetes mellitus | Yes | 39.47% | 0.001 |
| No | 12.90% | ||
| Age (yrs) | 20 | 4.57% | 0.004 |
| 40 | 12.27% | ||
| 60 | 28.96% | ||
| 80 | 54.31% | ||
| Duration of Symptoms before admission (days) | 1 | 12.19% | 0.003 |
| 2 | 14.83% | ||
| 3 | 17.93% | ||
| 4 | 21.50% | ||
| 6 | 30.11% | ||
| 8 | 40.39% | ||
| Dyspnea | Yes | 35.38% | <0.001 |
| No | 6.06% | ||
| Encephalopathy | Yes | 88.80% | <0.001 |
| No | 15.5% | ||
| Requirement of Oxygen therapy | Yes | 39.99% | <0.001 |
| No | 8.64% | ||
| Chest X -Ray findings | Normal | 1.42% | <0.001 |
| Unilateral opacities | 12.5% | ||
| Bilateral opacities | 54.34% | ||
| Total leucocyte count (/cmm) | 4000 | 8.01% | <0.001 |
| 8000 | 17.93% | ||
| 10000 | 25.71% | ||
| 12000 | 40.8% | ||
| 14000 | 46.48% |
Haemoglobin, Platelet count and electrolytes values were similar between both groups. However, The total leucocyte count was higher in the mortality group (11,059 ± 5,929 vs 7,022 ± 2,935, P = < 0.001). Radiologically, having bilateral opacities (wald χ2 (1) = 17.638, P < 0.001) or unilateral opacities (wald χ2 (1) = 3.653, P = 0.056) increased likelihood of mortality as compared to normal chest x-ray.
Discussion
Our institute is a Public Health Institute and was designated as Dedicated COVID Hospital from 18th April 2020. The COVID pandemic came with lockdown, cessation of public transport, fear factor (patients as well as Health Care Workers), closure of many dialysis centres, no known definitive treatment for COVID & resources crunch in the beginning. In addition, visiting dialysis center 2 to 3 times in a week for MHD increased the exposure risk of patients. In 36% of our cohort, regular MHD was less than thrice in a week HD. This coupled with missing one or more regular HDs during the lockdown period [before reporting to us] made these patients as one of the worst affected subgroups. This subset; is overburdened with co morbidities; as in our study population: Hypertension 76%, Diabetes 29%, Coronary Artery Disease 22% & underlying respiratory illness (COPD, post pulmonary koch's sequelae) 8%, & when COVID 19 infection (pneumonia with ARDS) complicates the course further, outcome is expected to be not favorable when compared with general population with COVID 19 infection.
Total 4324 patients with COVID 19 disease were admitted to our hospital during this period, with 203 requiring HD, of which 159 were ESRD & of these, outcome was noted in 131 during the study period. ESRD was reported in 3.2% at admission in 5,700 patients with COVID 19, admitted to 12 hospitals in New York.[11] CKD in 3% at admission was noted in cohort study with 1,591 patients with laboratory-confirmed COVID-19 admitted to intensive care units in Lombardy, Italy.[12] The Treatment protocols were evolving, changing, largely guided by the evidence at that time and hence cannot be co related with outcome. All ESRD patients were admitted & dialysed till their RT PCR turned negative. The average duration taken by patients to turn negative was 14 days (range 3-40 days).
One fourth of our study population was Asymptomatic. Asymptomatic patients have been reported to range from 1.6% to 56.5% in different studies.[13] Most notably, they took almost the same amount of time to convert to RT PCR negative as symptomatic patients. Since asymptomatic patients have the same infectivity,[14] if undetected, this subgroup of patients could potentially cause rapid spread of infection at dialysis centres. In a study from United Kingdom, clustering of cases in shifts and specific dialysis centres was commonly seen,[15] reiterating how dialysis centre could quickly turn into hotspots. Hence, strict social distancing, testing of all healthcare personnel and patients when clustering of cases occurs is advisable.
The management of COVID 19 disease in patients with CKD is more challenging given the immunosuppressed state & severe co morbidities. It is a severe disease in dialysis population. Overall mortality in China was reported at 2.3%.[1617] In Italy, the overall mortality & case fatality ratio, reported are, 20 per 1,00,000 & 12 respectively.[18] On the other hand, the mortality in dialysis population with COVID 19 infection has been reported at 31%[19] in New York and 30.5%[8] in Spain. The ESRD subgroup in our study group had a mortality rate of 20.61%. Comparing deceased vs survivors [Table 2 and Figure 1]: Age [56 vs 49 yrs], diabetes [56% vs 22%], Duration of symptoms at admission [5 vs 4 days] Dyspnea [85% vs 40%], oxygen requirement [93% vs 29%] and encephalopathy [30% vs 1%] at admission and high leucocyte count 11059 ± 5,929 vs 7,022 ± 2,935/cmm], Xray Chest with bilateral opacities [93% vs 20%] were statistically significant variables associated with mortality. Other factors like hypertension, coronary artery disease, underlying respiratory illness, dialysis vintage, dialysis frequency and access were not associated with increased mortality in our group.
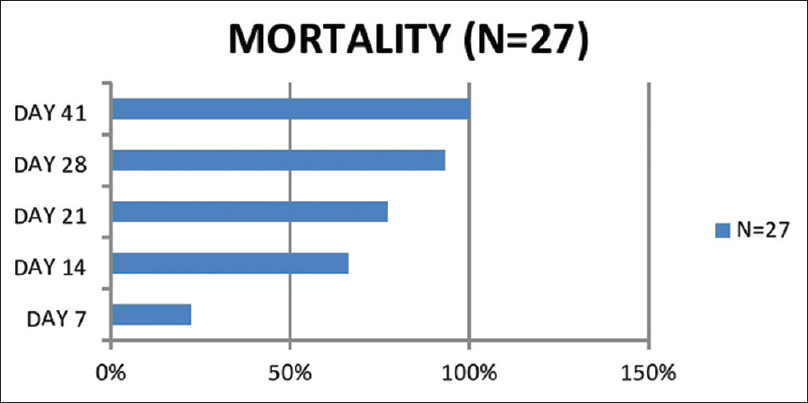
- Weekwise mortality. Among the deceased, 6 of 27 patients died within 1 week, 18 of 27 patients within 2 weeks, 21 of 27 patients within 3 weeks and 25 of 27 patients within 4 weeks. 1 patient died on day 37 and 1 patient on day 41 of disease
As compared to other countries, our ESRD patient population was younger with average age being 50 years & the likelihood of death with age more than 50 years, 60 yrs and 80 years was 19.28%, 28.96% and 54.31% respectively. The median Age was above 70 yrs in Italy[20] and Spain[8] and 63 yrs in a study from New york.[19] Their mortality rate was also much higher i.e., about 30% in European populations. High prevalence of hypertension (98%) and diabetes (69%) have been reported from New York[19] study.
Patients in the mortality group presented late to the centre for admission with higher number of patients having advanced symptoms like dyspnea, fever and altered sensorium. Happy hypoxia was another phenomenon commonly seen wherein patients had hypoxia requiring oxygen therapy but patient were relatively complain free or had mild symptoms only. Other factors like social stigma around the disease, difficulty in accessing healthcare and testing centres due to lockdown contributed to the late presentation.[21] Two third of the patients who died had ARDS on presentation.
An initial study from Wuhan[22] of 201 COVID19 patients from general population 93% patients had fever, 81% cough, 39.8% dyspnea and 41.8% patients developed ARDS, of whom 52.4% died. Subsequently, a meta-analysis[23] which included 19 studies and 39 case reports showed that fever was seen in 88.7%, cough in 57.6%, dyspnea in 45.6% patients and ARDS in 32.8%. Shock was seen only in 6.2%. Symptomatic fever (40%) and cough (33%) was seen much lesser in our ESRD population. Patients were at higher risk of developing ARDS. In our study 42% patients developed ARDS of whom 45.5% died. ARDS was also the most common cause of death. Diarrhoea was reported to be a common presenting symptom of covid19 in dialysis population in initial case reports,[24] however it was found in only 14.5% of our ESRD population.
Regular haemodialysis was also a challenge in initial days due to increasing number of patients needing emergency HD due to the reluctance of many outpatient dialysis centres to treat patients with COVID-19. The first 39 patients admitted with us were planned to be given twice weekly haemodialysis. However one third patients of these had to be shifted back to thrice weekly schedule due fluid overload and/or hyperkalaemia. One Hundred & four ESRD patients have been discharged after median of 21 days from onset of symptoms. Municipal Corporation of Greater Mumbai has created a software application & war rooms, wherein ESRD patients are registered by Nephrologists and COVID 19 infected patients are ensured vacant slots.
Limitations of study
Being tertiary care unit & dedicated COVID hospital, incidence of ESRD in admitted cases do not reflect incidence of ESRD in COVID 19 infection in Metro population. It is observational data. Given the pandemic situation and Mumbai being the epicentre in the early period, inflammatory markers and 2D echo could not be made available for all patients. Hence these details have not been analysed in present data. Further, no conclusions can be drawn regarding efficacy of drug therapy out of this study.
Conclusion
COVID 19 infection in hemodialysis population is severe disease. All due precautions should be taken to prevent infection in this vulnerable group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Dr Shruti Koley, Dr Vinod Chaudhary.
References
- Kerala confirmed first novel coronavirus case in India. India Today 2020 January 30
- [Google Scholar]
- “Home | Ministry of Health and Family Welfare | GOI”. mohfw.gov.in
- Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9.
- [Google Scholar]
- Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255–65.
- [Google Scholar]
- COVID-19 and kidney failure in the acute care setting: Our experience from Seattle. Am J Kidney Dis. 2020;76:4-6.
- [Google Scholar]
- 2019 novel coronavirus disease in hemodialysis (HD) patients: Report from one HD center in Wuhan, China. medRxiv doi: 10.1101/2020.02.24.20027201
- [Google Scholar]
- COVID-19: Clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98:27-34.
- [Google Scholar]
- Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31:1387-97.
- [Google Scholar]
- Guidelines for Dialysis with reference to COVID-19 Infection. Available from: https://www.mohfw.gov.in/pdf/GuidelinesforDialysisofCovid19 Patients.pdf
- Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–9.
- [Google Scholar]
- Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–81.
- [Google Scholar]
- A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect 2020 doi: 10.1016/j.jmii. 2020.05.001
- [Google Scholar]
- The epidemiological characteristics of infection in close contacts of COVID-19 in Ningbo city. Chin J Epidemiol. 2020;41:667-71.
- [Google Scholar]
- Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol. 2020;31:1815-23.
- [Google Scholar]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145-51.
- [Google Scholar]
- Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
- [Google Scholar]
- Case fatality rate analysis of Italian COVID-19 outbreak. J Med Virol. 2020;92:919-23.
- [Google Scholar]
- Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31:1409-15.
- [Google Scholar]
- A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98:20-6.
- [Google Scholar]
- Adding insult to injury: Kidney replacement therapy during COVID-19 in India. Kidney Int. 2020;98:238-9.
- [Google Scholar]
- Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1-11.
- [Google Scholar]
- Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid. 2020.101623
- [Google Scholar]
- COVID-19 in hemodialysis patients: A report of 5 cases. Am J Kidney Dis. 2020;76:141-3.
- [Google Scholar]







