Translate this page into:
Cyst-like Tumors in Renal Osteodystrophy
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Dear Editor
Chronic kidney disease (CKD) commonly evolves with disturbances in mineral and bone metabolism, currently defined as CKD-mineral bone diseases. The range of skeletal abnormalities in renal bone disease encompasses syndrome with high and low turnover. Secondary hyperparathyroidism is a frequent complication of CKD with high bone turnover. Elevated levels of PTH in blood and hyperplasia of parathyroid glands are seen early in the course of the renal failure. Osteitis fibrosa (OF), the most frequent type of renal osteodystrophy, is a skeletal disorder characterized by sustained hyperparathyroidism, high bone turnover, usually low bone mineral density, increased osteoclast/osteoblast activity, peritrabecular fibrosis and rarely formation of cyst-like tumors in the bone. Skeletal deformities, bone pain and pathological fractures are the most common features of OF. Due to better management of CKD, the advanced findings of OF are no longer commonly seen. Routine radiologic imaging of the skeleton is relatively insensitive for the diagnosis of renal osteodystrophy. Subperiosteal resorption detected in the hands, clavicles and pelvis; focal radiolucency and a ground-glass appearance (pepper pot) in the skull; “rugger-jersey” appearance of the spine are the most reported findings in OF. Cyst-like tumors, also called “brown tumors”, are uncommon. The lesions are localized in areas of intense bone resorption (facial skeleton, clavicle, ribs and pelvic bones). Brown tumors, also known as osteoclastoma, are associated with hyperparathyroidism in the renal osteodystrophy. They are usually seen as well-demarcated radiolucent zones in long bones, clavicles and digits. CT scan characteristics of brown tumor are expansile lytic lesions, with various amounts of bone destruction and thinning of trabecula. These lesions are usually benign but in renal OF can lead to severe complications.[12345] The name “brown tumor” derives from the color, which is caused by hypervascularity, hemorrhage and deposits of hemosiderin. A 55-year-old Caucasian male developed ESRD at the age of 46 due to hypertensive disease. In this period, he had a normal value of PTH, high phosphate and low calcium serum levels without signs of abnormalities on clinical examination (cervical mass) and imaging (renal stones, bone lesions). He was treated with CAPD. During the following 3 years the patient developed gradually increasing PTH levels to 1946 pg/ml, primarily because he refused to take any oral medication such as phosphate-binders, calcitriol or cinacalcet. Also, frequently the patient had poor compliance with his diet and dialysis schedule. The patient had high phosphate and low calcium serum levels. Based on the medical history and PTH, phosphate and calcium laboratory tests, we excluded a primary hyperparathyroidism and we made diagnosis of secondary hyperparathyroidism. In 2019 the patient began to describe pain in back and left hip and inability of walking. He had no history of trauma. Physical examination showed thoracic kyphosis and a soft mass in her left hip. Ultrasonography of the neck revealed multiple parathyroid adenomas. The patient refused the nuclear parathyroid scintigraphy, histological examination of parathyroid adenoma and finally the parathyroidectomy. CT scan showed patches of osteolysis in the skull, the ribs, right clavicle, right and left pubis and a wide cystic lesion in the left iliac bone. [Figure 1] The spine showed a rugger jersey finding, osteolytic lesions and a subchondral resorption on the symphysis pubis. [Figure 2] The skull showed mixed lytic and sclerotic lesions with punctate lucent foci [Figure 3]. We performed urologic evaluation with exclusion of prostatic abnormalities and contrast-enhanced CT scan did not highlight any visceral findings. The laboratory workup (serum protein electrophoresis, immunofixation electrophoresis, serum-free light chains quantification) did not reveal any sign of plasma cell Dyscrasia. The clinical examination and imaging findings could exclude systemic diseases leading to osteolytic lesions. The differential diagnosis includes Langerhans cell histiocytosis, aneurysmal one cyst, multiple myeloma, giant cell tumor, Paget's disease and metastasis. We lost the patient to follow-up.
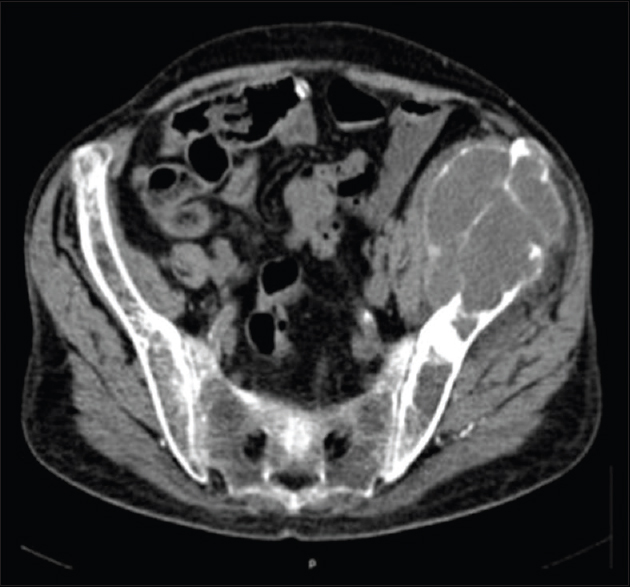
- Axial CT scan of the pelvis showing lytic lesion in the spine and wide exophytic brown tumor-like lesion (7.5 cm × 6.5 cm) in the left iliac bonewith regular borders and thinned cortical bone
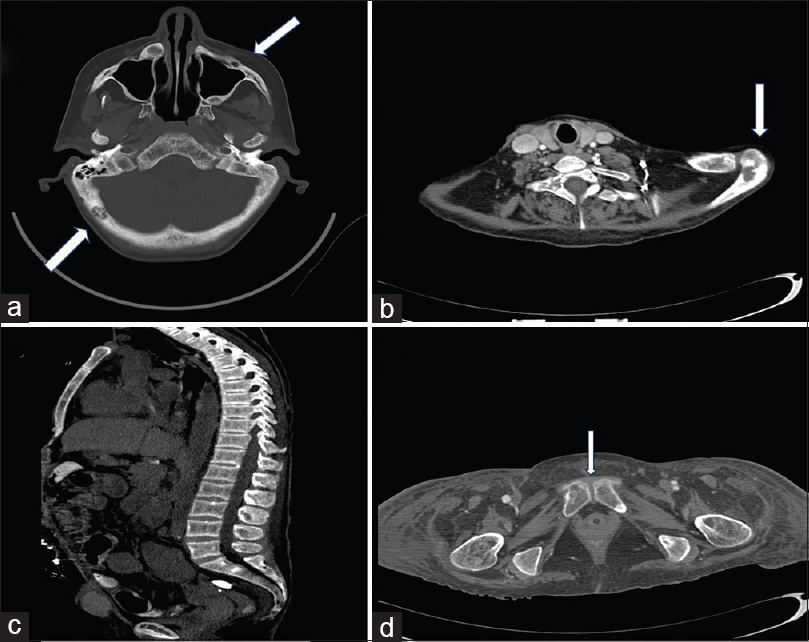
- (a) Mixed lytic and sclerotic lesions in the skull and right maxillary bone; (b) Lytic lesions in the lamina and left scapula; (c) Rugger jersey spine; (d) Subchondral resorption in the symphysis pubis
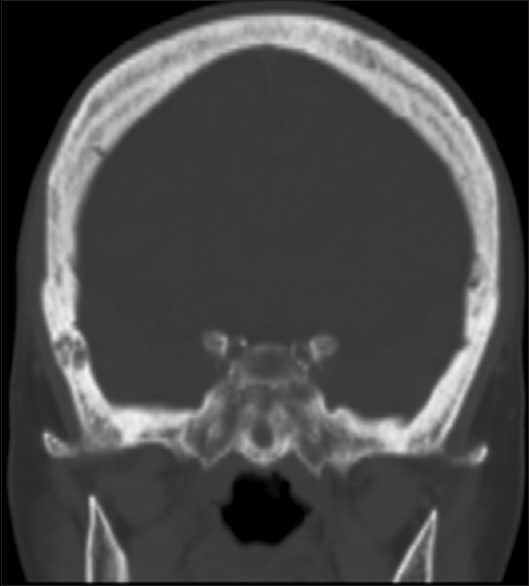
- Mixed lytic and sclerotic lesions with punctate lucent foci: Salt-and pepper appearance
Financial support and sponsorship
The Authors do not report any financial support and sponsorship.
Conflicts of interest
The Authors do not report any conflict of interest.
References
- Multiple brown tumors-a rare presentation in the modern era. Clin Nephrol. 2018;89:57-60.
- [Google Scholar]
- Brown tumor of secondary hyperparathyroidism: Surgical approach and clinical outcome. Oral Maxillofac Surg. 2016;20:435-9.
- [Google Scholar]
- Brown tumor of the cervical spines: A case report with literature review. Asian Spine J. 2015;9:110-20.
- [Google Scholar]
- Multiple bone brown tumor secondary to primary hyperparathyroidism: A case report and literature review. Gland Surg. 2019;8:810-6.
- [Google Scholar]
- Management of brown tumor of spine with primary hyperparathyroidism: A case report and literature review. Medicine (Baltimore). 2019;98:e15007.
- [Google Scholar]






