Translate this page into:
ABO-Incompatible Kidney Transplantation in India: A Single-Center Experience of First Hundred Cases
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim:
ABO-incompatible (ABOi) kidney transplantation overcomes immunological barrier of blood group incompatibility. There have been very few published experiences of ABOi kidney transplantation from India. We present our single-center experience of the first hundred ABOi kidney transplants.
Material and Methods:
This is a single-center retrospective study of consecutive first hundred ABOi kidney transplant with at least 6 months of follow-up.
Results:
During the study period (2011–2020), a total of 121 ABOi kidney transplants were performed. Of these, first hundred patients were analyzed. Median follow-up duration was 33 (10–101) months. Mean recipient and donor age were 41.5 ± 13 and 47.68 ± 11.25 years, respectively. Mean HLA mismatch was 4 ± 1.5. Median baseline anti-blood group antibody titer was 128 (2–1024). Most common recipient blood group was O. Patient and death censored graft survival was 93% and 94%, respectively, at median follow-up of 33 months. Biopsy-proven acute rejection (BPAR) rate was 17% with acute antibody-mediated rejection being 3%. Rate of infection was 37%, most common being urinary tract infection.
Conclusion:
ABOi kidney transplant patients had acceptable patient and graft survival as well as BPAR rates. With current preconditioning protocol, infection rate was high.
Keywords
Antibodies
blood group incompatibility
India
kidney transplantation
living donors
Introduction
Blood group incompatibility is an important immunological barrier in kidney transplantation. The use of plasmapheresis as a method to remove isoagglutinin was a watershed moment in the history of ABO incompatible (ABOi) kidney transplant.[1] Alexandre et al. from Belgium were the first to report a successful series of ABOi transplants with the use of plasmapheresis and splenectomy.[2] Japanese used it widely in the late 1980s followed by Americans and Europeans to increase the donor pool.[345] Use of rituximab in the early 2000s replaced surgical splenectomy, which used to be associated with high morbidity and mortality.[6] Last few decades have witnessed significant improvement in the outcomes of ABOi kidney transplant with the advent of newer preconditioning protocols and improved maintenance immunosuppression. There are very few publications of ABOi kidney transplants from the developing world. In the present study, we present our single-center experience of first hundred ABOi transplants performed over the last decade.
Materials and Methods
This was a single-center retrospective study performed at a tertiary health-care center in India. First ABOi kidney transplant at our center was performed in November 2011. Since then, 121 such transplants have been performed. First hundred consecutive ABOi kidney transplants performed were included for this study. All these patients had at least 6 months of follow-up.
Pretransplant evaluation
Kidney transplant evaluation for donor and recipient was done as per the center protocol. IgG and IgM antibody titer against donor ABO blood group antigens were tested in patient's serum using column agglutination technology with low-ionic-strength saline-indirect antiglobulin test technique (Ortho-Clinical Diagnostics, Johnson and Johnson, USA). The cassettes used were antihuman globulin type. This method of antibody titer determination also known as the gel method is known to be more sensitive than the conventional tube test. Immunological tests included HLA typing of donor and recipient and complement-dependent cytotoxicity and flow cytometry cross-matches.
Preconditioning protocol
Preconditioning protocol has evolved at our center since the first ABOi transplant. The first patient had an isoagglutinin titer of 512. He had received 500 mg of IV rituximab. For subsequent transplants, the dose of rituximab was reduced to 200 mg, as there were reports of successful outcomes with reduced dose as well. Rituximab was given tentatively 2 weeks before the scheduled transplant. One week after rituximab administration, the patient was admitted for extracorporeal isoagglutinin (anti-blood group antibodies) removal. In the initial few patients, this was done by regular plasmapheresis. Later, cascade plasmapheresis (CP) or immunoadsorption (IA) were used in view of advantages such as better tolerability and safety profile. We evaluated and published our experience of CP versus regular plasmapheresis. We found that CP was safe and effective with acceptable clinical outcomes. The average number of sessions required in regular plasmapheresis to achieve the desired titer was higher when compared to CP with lesser requirement of plasma infusions.[7] In our experience, we also found that lesser number of IA sessions are required to achieve the desired target although it is not cost effective when the titer is <128 when compared to CP. Five gram of IVIG was given after each session of CP or plasmapheresis but not after IA. Variability of anti-blood group antibody titer in different batches of IVIG products has been shown. If patient receives a batch with high anti-blood group antibody titer, it can lead to rebound of titer and thereby delay in achieving the desired target.[8] Hence, lately we have restricted the use of IVIG in special circumstances such as HLA sensitizations or those undergoing multiple plasmapheresis sessions. While giving IVIG, we are careful to select the batch with minimum anti-blood group antibody titer. Extracorporeal isoagglutinin removal was performed on alternate days till the titer reduced to 8 at which point kidney transplant was done. Patient was started on tacrolimus (0.05 mg/kg in two divided doses bid) and mycophenolate sodium (720 mg bid) 1 week before tentative transplant. A tacrolimus trough level of 8–12 ng/ml was targeted pretransplant. There were nine patients with baseline antibody titer of ≤8 who did not require extracorporeal isoagglutinin removal.
Intraoperatively 500 mg of IV methylprednisolone was given. Induction used was basiliximab, polyclonal antihuman T-lymphocyte immunoglobulin (Grafalon©, Neovii Pharmaceuticals AG, Switzerland – formerly known as ATG-Fresenius or ATG-F) or anti-thymocyte immunoglobulin (Thymoglobulin©, Sano-Aventis, Boston, USA) depending upon sensitization status (sensitized patients with the history of blood transfusion, pregnancies, second transplant or a positive cross-match received grafalon or thymoglobulin induction). Oral prednisolone was started at 40 mg/day from the next day of transplant and was tapered to 20 mg/day on discharge. Isoagglutinin titer was monitored daily till discharge and posttransplant CP was done only in case of rising titer with accompanying graft dysfunction while awaiting graft biopsy.
Patients were followed up weekly twice for the first month, weekly once for the second month, once in a fortnight for the 3rd month and monthly thereafter for the first year. After the first year, patient was followed up once in 2–3 months. Tacrolimus target trough level was 8–12 ng/ml during first 3 months, 5–8 ng/ml from 3 to 6 months, and <5 ng/ml thereafter. Prednisolone was tapered to 5 mg by the end of 3rd month.
Graft biopsy was done in case of graft dysfunction or other indications such as proteinuria. Initial nine patients underwent protocol graft biopsy. Subsequently, protocol graft kidney biopsy was discontinued due to unwillingness of patients.
Statistical analysis was done using Prism GraphPad for Mac, version 8 (GraphPad Software, San Diego, California). Data were reported as mean values ± standard deviation. Continuous variables were compared using unpaired t-test while categorical values were compared using Chi-square test or Fisher's exact test. Survival analysis was performed by the Kaplan–Meier method and groups were compared using the log-rank test. P < 0.05 was considered as statistically significant.
The study was approved by the local ethics committee of the hospital (MICR 1102/2020).
Results
Since November 2011 till March 2020, a total of 121 ABOi kidney transplants were performed. First hundred patients who completed at least 6 months of follow-up were included. Median follow-up duration was 33 (10–101) months.
Table 1 shows baseline characteristics of the patients. Most common recipient blood group was O. The median baseline isoagglutinin titer was 128 (range 2 to 1024). Three patients were second transplant recipients. Two patients were both ABO and HLA-incompatible kidney transplant. Donor relation was wife in 51%, mother in 26%, siblings in 7%, father in 6%, husband in 3% and others in the remaining 7%. No induction was used in 13 patients while basiliximab was used in 65 patients, thymoglobulin in 11 patients, and grafalon in 11 patients.
| Variable | Value (n = 100) |
|---|---|
| Average recipient age (years) | 41.5 ± 13 |
| Average donor age (years) | 47.68 ± 11.25 |
| Recipient gender | 81% M; 19% F |
| Donor gender | 17% M; 83% F |
| Median dialysis vintage (months) | 5 (0.5-84) |
| Preemptive transplants | 12 (12%) |
| HLA mismatch | 4 ± 1.5 |
| Blood group distribution | |
| AB to A | 7 (7%) |
| AB to B | 15 (15%) |
| A to O | 28 (28%) |
| B to O | 22 (22%) |
| AB to O | 1 (1%) |
| B to A | 14 (14%) |
| A to B | 13 (13%) |
| Antibody titer distribution | |
| 1:2 | 1 (1%) |
| 1:4 | 3 (3%) |
| 1:8 | 5 (5%) |
| 1:16 | 10 (10%) |
| 1:32 | 10 (10%) |
| 1:64 | 14 (14%) |
| 1:128 | 13 (13%) |
| 1:256 | 21 (21%) |
| 1:512 | 19 (19%) |
| 1:1024 | 4 (4%) |
M = Male; F = Female; HLA = Human leucocyte antigen
Patient outcome is shown in Table 2. Patient survival was 93%. There were seven mortalities, of which four were due to infection and three due to cardiac event. One patient expired after 41 months of graft loss. Other patients expired with a functioning graft. Death censored graft survival was 94%. Figure 1 shows Kaplan–Meier curves for patient and graft survival. Biopsy-proven acute rejection rate was 17%. Rate of acute antibody-mediated rejection was 3%.
| Variable | Value (n = 100) |
|---|---|
| Patient survival at median follow-up of 33 (10-101) months | 93 (93%) |
| Cause of patient loss | |
| Infection | 4 |
| Acute coronary syndrome | 3 |
| Death censored graft survival at median follow-up of 33 (10-101) months | 94 (94%) |
| Cause of death censored graft loss | |
| Acute antibody-mediated rejection | 3 |
| Renal vein thrombosis | 1 |
| Chronic thrombotic microangiopathy | 1 |
| Chronic AMR due to drug non-compliance | 1 |
| Biopsy-proven acute rejection at median follow-up of 33 (10-101) months | 17 (17%) |
| Types of biopsy proven acute rejection | ACR-14 (14%); AMR-3 (3%) |
| Serum creatinine (µmol/l) | |
| 1 month | 108 ± 40 |
| Last follow-up - at median follow-up of 33 (10-101) months | 107 ± 37 |
| NODAT at median follow-up of 33 (10-101) months | 15 (15%) |
| Infection at median follow-up of 33 (10-101) months | 37 (37%) |
ACR = Acute cellular rejection; AMR = Antibody-mediated rejection; NODAT = New-onset diabetes after transplantation

- Kaplan–Meier graph for (a) patient survival and (b) graft survival. The number of transplants done in first 3 years was 7 only, followed by 37 in next 3 years and 56 in last 3 years
Thirty-seven percentage patients had 53 episodes of infection. Most common of them was urinary tract infection (UTI) followed by cytomegalovirus (CMV) infection. Figure 2 shows the details of these infections.
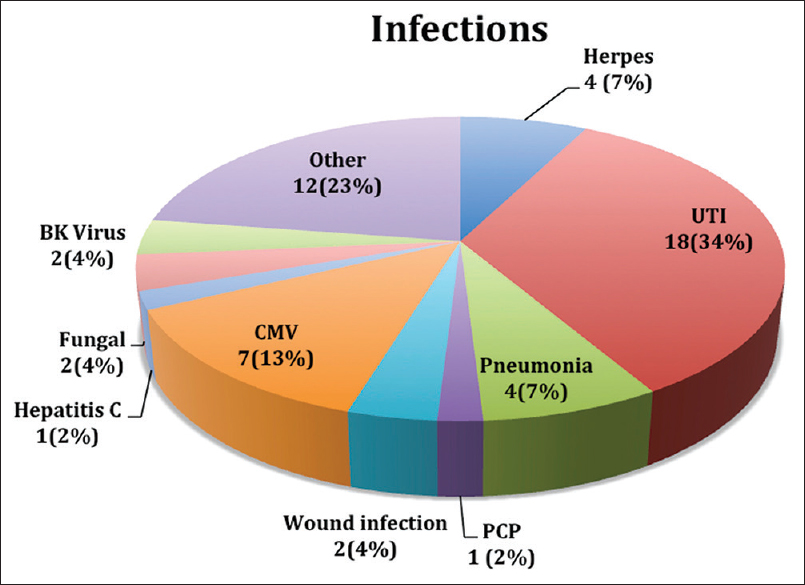
- Distribution of infections in ABO incompatible recipients. There were total 53 episodes of infections in 37 patients
Discussion
ABOi kidney transplantation has come a long way with initially being a contraindication for transplant to now contributing to up to one-third of kidney transplant in countries like Japan.[9] The popularity has increased over the last few decades with improving understanding of the immunological mechanisms underlying hyperacute rejection in such transplants, phenomenon of accommodation, and the availability of newer immunosuppressive medications.
The inadequate penetration of deceased donor program and lack of a national level registry data have led to increased acceptance of ABOi as a viable alternative in India as well. We published the first case report of ABOi kidney transplant from India.[10] From 2011 till 2013, a total of 7 ABOi transplants were done. As we gained more experience and confidence, this number increased to 37 during the period 2014–2016 and 56 during 2017–2019. Subsequently, we also published our initial experience of first 20 ABOi at our center with short follow-up.[11] The present article is first single-center experience of hundred ABOi kidney transplants from India. The age and sex distribution of our ABOi kidney transplant were comparable to our compatible transplants in general. The baseline titer varied from 2 to 1024 with median titer being 128. Nine patients had titer ≤8.
Patient survival was 93% in the present study. One-year patient survival was 98% in a meta-analysis by de-Weerd et al. while 5-year patient survival in UK national registry data of ABOi kidney transplant was 91%.[1213] In a large US national multicenter data, patient survival was 93.7% at 3 years and 88.3% at 5 years.[4] Major cause of patient loss in the present study was infection. In the study by de-Weerd et al., the cause of patient mortality was infection in 49% of cases.[12] Similarly, the collaborative transplant study also showed higher infection-related mortality in ABOi transplants.[5] Graft survival in our study was 94%. In the study by de-Weerd et al., one-year uncensored graft survival was 96% while in that of study by Montgomery et al., it was 82.6% at 3 years.[412] Major cause of graft loss in our study was acute antibody-mediated rejection (AMR).
In the present study, biopsy-proven acute rejection rate was 17%. The acute AMR rate was 3%. All these AMRs happened within initial 72 h and biopsy findings were remarkable for the presence of thrombotic microangiopathy (TMA) in all of them. Single antigen bead by Luminex did not reveal any anti-HLA antibody in at least two of them in whom it was done post-rejection. Similar findings of TMA and AMR have been reported in other studies as well and eculizumab has been successfully used to reverse such AMRs in few cases.[141516] The proposed mechanism of such TMA is endothelial damage caused by anti-blood group antibodies activating the complement system. In a study by Tasaki et al., nonuse of mycophenolate mofetil, pretreatment immunoglobulin G antibody titer ≥64- and pretransplant immunoglobulin M antibody titer ≥16- were significant risk factors for TMA in ABOi transplants.[15] None of the AMRs in our patients could be reversed despite prompt plasma exchange, IVIG, and other antirejection treatment such as thymoglobulin. We could not use eculizumab due to its nonavailability in India. All the episodes of acute AMR in our patients lead to the graft loss and ultimately required graft nephrectomy. Apart from these patients with acute TMA, another patient had chronic TMA leading to graft loss. Miura et al. have reported a similar case of chronic TMA causing graft loss in an ABOi transplant.[17]
Rate of infection was 37% in the present study. Infection has been an area of concern in previous studies of ABOi transplants as well, which has been thought to be due to extra immunosuppressive burden in these patients. Opelz et al. in a multicenter study of 1420 ABOi patients across Europe found that 1 additional death per 100 patients occurred in the first year after ABOi transplants when compared to ABOc patients.[5] Higher rates of nonviral and viral infections were also seen in the meta-analysis done by de-Weerd et al.[12] In our study, UTI was the major infection followed by CMV. Increase in viral infections such as CMV and BK virus (BKV) has also been reported in previous studies as well.[121819]
Limitations of the present study include relatively short duration of follow-up and retrospective design of the study. Also, there was heterogeneity in the use of induction. Despite these limitations, this is an important study because of the relatively large sample size compared to the previous studies from India.
ABOi transplant is an evolving field, and with increasing experience, the outcomes are improving as well. The Indian working group on ABOi renal transplant published guidelines, which has provided recommendations in this regard. This answers some important questions such as what is the best method of antibody determination, what should be the acceptable pretransplant titer, which is the best preconditioning protocol and what is the role of rituximab and IVIG in such transplants.[20]
Concluding, ABOi kidney transplant is a good alternative for patients who do not have suitable blood group compatible donors. The short-term patient and graft survival are good. There is an increased risk of infection in such transplants, and hence there is a need to be aggressive in minimizing the immunosuppression in these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Vijay Kher has been in the speaker forum for Zydus as well as Genzyme/Sanofi and has also received research grants from Genzyme. Dr. Ajay Kher has been in the speaker forum for Sanofi. Other authors do not have any conflicts of interest associated with this publication.
References
- Renal transplant in a patient with major donor-recipient blood group incompatibility: Reversal of acute rejection by the use of modified plasmapheresis. Transplantation. 1981;31:4-7.
- [Google Scholar]
- Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. 1987;19:4538-42.
- [Google Scholar]
- Long-term results of ABO-incompatible living kidney transplantation: A single-center experience. Transplantation. 1998;65:224-8.
- [Google Scholar]
- Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93:603-9.
- [Google Scholar]
- Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: Results from 101 centers. Transplantation. 2015;99:400-4.
- [Google Scholar]
- Successful ABO-incompatible kidney transplantations without splenectomy using antigen-specific immunoadsorption and rituximab. Transplantation. 2003;76:730-1.
- [Google Scholar]
- Cascade plasmapheresis as preconditioning regimen for ABO-incompatible renal transplantation: A single-center experience. Transfusion. 2016;56:956-61.
- [Google Scholar]
- Anti A/B antibody titer rebound: Are we making it worse. Be aware of your intravenous immunoglobulin? Indian J Nephrol. 2018;28:407-9.
- [Google Scholar]
- ABO-incompatible living kidney transplants: Evolution of outcomes and immunosuppressive management. Am J Transplant. 2016;16:886-96.
- [Google Scholar]
- ABO-incompatible renal transplantation in developing world - crossing the immunological (and mental) barrier. Indian J Nephrol. 2016;26:113-8.
- [Google Scholar]
- ABO-Incompatible kidney transplant outcomes: A meta-analysis. Clin J Am Soc Nephrol. 2018;13:1234-43.
- [Google Scholar]
- The UK national registry of ABO and HLA antibody incompatible renal transplantation: Pretransplant factors associated with outcome in 879 transplants. Transplant Direct. 2017;3:e181.
- [Google Scholar]
- Eculizumab for thrombotic microangiopathy associated with antibody-mediated rejection after ABO-incompatible kidney transplantation. Case Rep Transplant. 2017;2017:3197042.
- [Google Scholar]
- Analysis of the prevalence of systemic de novo thrombotic microangiopathy after ABO-incompatible kidney transplantation and the associated risk factors. Int J Urol. 2019;26:1128-37.
- [Google Scholar]
- Hyperacute onset of haemolytic-uraemic syndrome associated with hyperacute rejection in the recipient of an ABO-incompatible kidney. Nephrol Dial Transplant. 2003;18:1009-12.
- [Google Scholar]
- A case of progressive thrombotic microangiopathy after ABO-incompatible renal transplantation. Clin Transplant. 2011;25(Suppl 23):19-22.
- [Google Scholar]
- Outcomes and complications following ABO-incompatible kidney transplantation performed after desensitization by semi-selective immunoadsorption - a retrospective study. Transpl Int. 2019;32:1286-96.
- [Google Scholar]
- Increase of infectious complications in ABO-incompatible kidney transplant recipients--a single centre experience. Nephrol Dial Transplant. 2011;26:4124-31.
- [Google Scholar]
- ABO-incompatible kidney transplantation: Indian working group recommendations. Indian J Transplant. 2019;13:252-8.
- [Google Scholar]







