Translate this page into:
Hypervitaminosis D and Acute Interstitial Nephritis: Tale of Injections
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A 33-year-old man came with nausea, vomiting and abdominal pain due to hypercalcaemia and renal dysfunction following two doses of intramuscular vitamin D injections. Levels of vitamin D were repeatedly above 300 ng/ml over a period of 10 months. Whole-body PET CT scan revealed a thin-walled collection in the right gluteal region. The patient refused a surgical intervention for the same. After 7 months of follow-up, the abscess ruptured spontaneously and was then surgically debrided. At this point, a history of pentazocine addiction was uncovered. One month later, vitamin D levels began to fall along with improvement in serum calcium and creatinine. This case unravels a diagnostic odyssey which ended with a simple surgical debridement. We aim to highlight that vitamin D supplementation in ‘megadoses’ in the presence of active infection can have an exaggerated response and may take months to resolve.
Keywords
AKI
AIN
gluteal abscess
hypervitaminosis D
Introduction
Vitamin D is much more than a mere fat-soluble vitamin. Its pleiotropic actions have touched almost all physiological processes. According to various studies, around 80–90% of Indians are vitamin D deficient.[123] Due to its wide therapeutic window many times, it is given empirically. Its easy availability as an over-the-counter medicine sometimes leads to inadvertent or excessive using. Though generally considered safe, vitamin D toxicity does occur. We hereby report a case of iatrogenic vitamin D toxicity, with an unusually prolonged course of illness following an apparently standard dosage.
Case
A 33-year-old male presented in emergency with nausea, vomiting, vague dull aching abdominal pain and lethargy for past 2 weeks. He gave a history of generalized weakness, low-grade intermittent fever and decreased appetite with weight loss of about 10 kg over past 6 months. He was told to have vitamin D deficiency (actual values not available) and had received intramuscular vitamin D in gluteal region, two doses, 1 week apart, 10 weeks prior to presentation. Skin over the gluteal regions showed areas of thickening and hyperpigmentation. The initial investigations are shown in Table 1. A provisional diagnosis of hypervitaminosis D and ischemic and/or hypercalcaemia-related renal dysfunction with pyrexia of unknown origin was made. Aggressive intravenous hydration followed by diuretics and intranasal calcitonin along with empiric antibiotics were initiated. Over the next 3 days, patient improved symptomatically and was afebrile. However, hypercalcaemia persisted; hence, further workup was planned to rule out other aetiologies of hypercalcaemia as shown in Table 1. Non-contrast FDG PETCT [Figure 1] revealed thin-walled loculated collections in the right gluteal region. Patient was advised a surgical debridement which he refused and chose to continue conservative treatment alone. An ultrasound-guided aspiration of gluteal collection was performed and sent for stain and cultures (bacterial, tubercular and fungal), which subsequently came negative. Empiric parenteral antibiotics were continued for 14 days. A repeat MRI after 2 weeks showed partial resolution of the collection in the right gluteal region. His creatinine levels kept fluctuating between 2.0 and 2.5 mg/dl. In view of persistent renal dysfunction, a kidney biopsy was done which showed acute interstial nephritis with acute tubular necrosis [Figure 2]. After a four-week trial of oral corticosteroids (along with oral antibiotics), there was neither improvement in creatinine nor calcium. He was administered a single dose of denosumab 60 mg subcutaneously. Genetic testing was done to rule out underactive CYP24A1 variants. No pathogenic mutations were found. He was advised further investigations; however, he was lost to follow-up.
| Investigations | At admission | At follow-up (after 10 months) |
|---|---|---|
| Hb, g/dl | 7.8 | 10.4 |
| TLC, ×103 cells/ml | 8.4 | 5.6 |
| CRP, mg/l | 78 | 12 |
| Serum creatinine, mg/dl | 2.6 | 1.47 |
| Calcium, mg/dl | 12.14 | 9.2 |
| Phosphorus, mg/dl | 2.28 | 3.45 |
| Total protein, g/dl | 6.04 | 6.7 |
| Alkaline phosphatase, IU/l | 109 | |
| Serum albumin, g/dl | 2.59 | 3.8 |
| 25(OH) vitamin D, ng/ml | >300 | 111 |
| 1,25(OH)2 vitamin D, pg/ml | >185 | 40 |
| iPTH, pg/ml | 6.2 | 34 |
| PTHrP, pg/ml | 12 | - |
| ACE levels, IU/l (normal <55) | 35 | - |
| Serum protein electrophoresis | No M band | |
| Free light chain assay (Kappa/Lambda ratio) | 0.9 | |
| Urine microscopy | Calcium oxalate crystals seen | |
| 24 h Urine calcium (100-300 mg/day) | 474.71 | - |

- FDG PET CT scan in coronal view showing mild FDG-avid thin-walled loculated collection in the right gluteal region (white arrow)

- Photomicrograph showing tubular calcifications (black arrow), focal acute tubular necrosis (ATN) with normal glomerulus. No granuloma or eosinophils were evident on renal biopsy (H and E, ×100)
He presented for the second time to our institute 7 months later with a spontaneously ruptured gluteal abscess after having received oral steroids prescribed at a local hospital. The vitamin D levels continued to be high. An urgent surgical exploration was done with drainage of around 500 ml of pus from gluteal region and cultures (bacterial, fungal and tubercular) were sent, which came out negative. On enquiry, he disclosed the abuse of intramuscular injection pentazocine, multiple doses over previous 2 years, first of which was given for abdominal pain.
One month after the debridement surgery, vitamin D levels began to show a downward trend along with normalization of serum calcium and improvement of renal function [Figures 3, 4 and Table 1]. He is now on regular deaddiction sessions and has reported no further injectable administration for last 3 months.
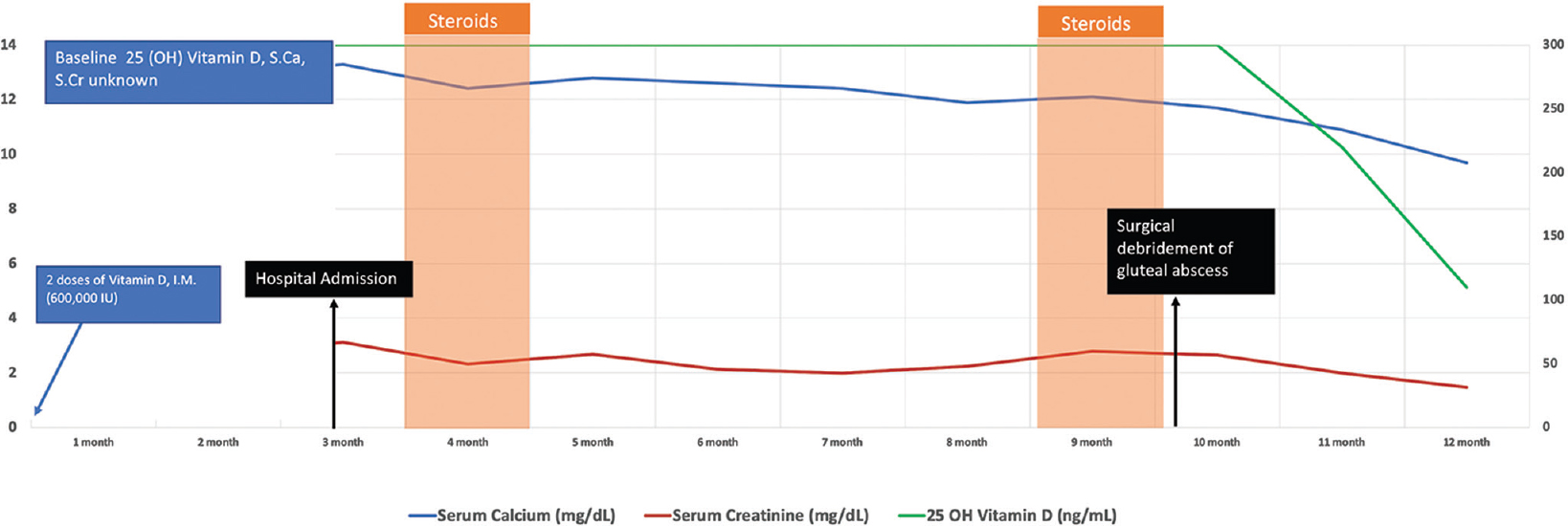
- Diagram showing the clinical course of the patient over 1 year after the administration of Vitamin D injection. S.Ca: Serum calcium, S.Cr: serum creatinine
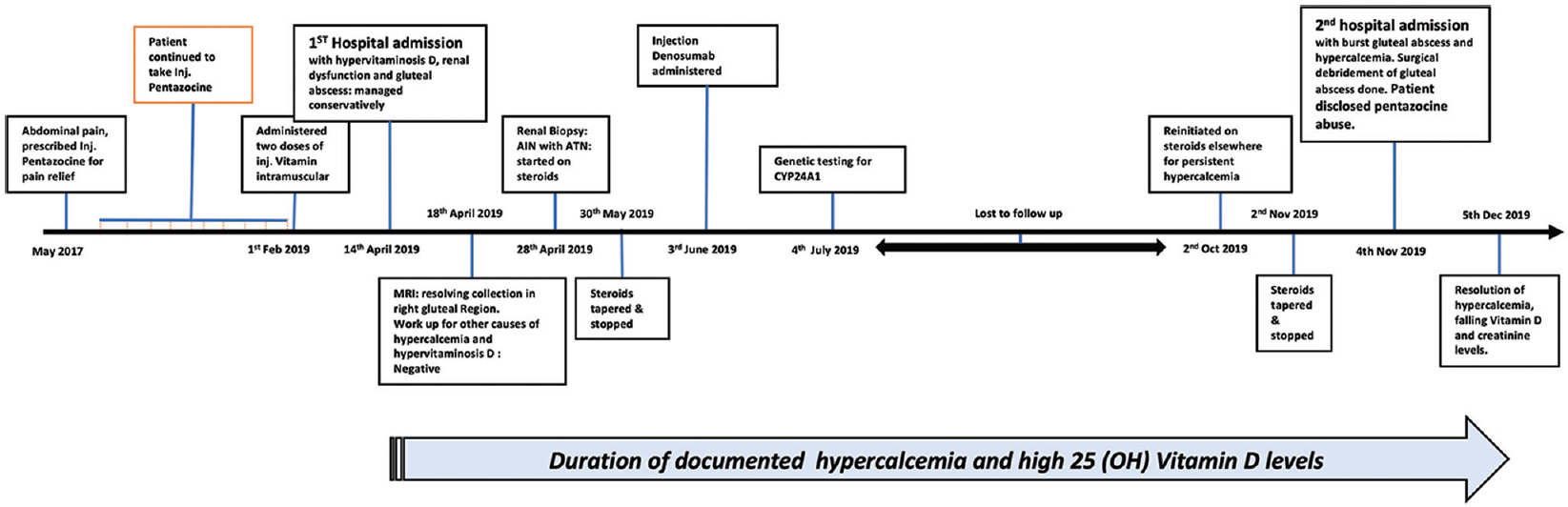
- Timeline depicting the significant turn of events in the clinical course of the patient
Discussion
Various trials have proved the safety and efficacy of the ‘megadose’ of vitamin D (600,000 IU).[45] Although vitamin D is believed to have a wide therapeutic index, cases of hypervitaminosis D have been reported at levels lower than this. In a case series from India, by Misgar et al., vitamin D toxicity has been reported with doses ranging from 180,000 to 420,000 IU.[6]
The various causes of hypervitaminosis D are shown in Table 2. The levels of 1,25(OH)2 vitamin D in iatrogenic vitamin D toxicity (VDT) are variable. They can be slightly high or normal.[78] The mechanism of toxicity of hypervitaminosis D is not entirely understood. As most studies did not find high 1,25(OH)2 vitamin D levels in patients with VDT, it is proposed that 25(OH) vitamin D itself in higher concentrations stimulates vitamin D receptors. In our case, both 25 and 1,25(OH)2 vitamin D were high, which is uncommon and is possibly due to continuous conversion of 25(OH) Vitamin D to 1,25(OH)2 vitamin D and its release from the reservoir of excess vitamin D administered intramuscularly.
| Condition | 25(OH) vitamin D | 1,25(OH)2 vitamin D | PTH | S.Ca2+ | ALP |
|---|---|---|---|---|---|
| Exogenous | |||||
| 25(OH) vitamin D supplement | ↑ | N/↑ | ↓ | ↑ | N/↓ |
| Activated vitamin D, vitamin D analogues | N/↓ | ↑ | ↓ | ↑ | N/↓ |
| Endogenous | |||||
| Granulomatous disease (e.g. sarcoidosis, TB) | N/↓ | ↑ | ↓ | ↑ | N/↓ |
| Lymphoma | N/↓ | ↑ | ↓ | ↑ | N/↑ |
| CYP24A1 mutation | ↑ | ↑ | ↓ | ↑ | N/A |
Vitamin D is a fat-soluble vitamin, and adipose tissue is the predominant site of vitamin D storage. After the intake, it is rapidly distributed into adipose tissue and slowly released into the circulation. This acts as a protective mechanism against the rapid release of vitamin D into the system and causing hypervitaminosis D.[910] The relationship between weight loss and vitamin D has also been evaluated in the past.[1112] Adiposity is inversely related to 25(OH) vitamin D, and obese people have lower 25(OH) vitamin D levels.[10] Our patient had a weight loss of around 16 kg over a period of 18 months (BMI decreased from 27.3 to 21.6). Suppressed appetite, poor oral intake and volume depletion due to hypercalcaemia further compounded by catabolism of chronic inflammation lead to significant weight loss. This weight loss could be one of the explanations for a prolonged duration of high levels of 25(OH) vitamin D levels in circulation despite the cessation of further exposure. In a study by Lin et al., they studied the effect of weight loss after bariatric surgery and 25(OH) vitamin D levels. They observed a positive association between adipose tissue mass and 25(OH) vitamin D levels concluding that it is released during weight loss.[12] The other explanation, in this case, maybe the injection of vitamin D into the abscess (formed due to the prior pentozocine injections) that served as a reservoir/storage for the continuous release of vitamin D over months. The temporal association of normalization of calcium levels and fall in vitamin D levels after complete drainage of gluteal abscess supports this theory. Previously published studies have reported that hypervitaminosis D can last from a few days to several months. In a study by Martinaityte et al., it was shown that vitamin D is stored in adipose tissue and can have a terminal half-life of 255 days after prolonged supplementation.[13] In the case series of 32 patients with vitamin D toxicity by Misgar et al., the duration of hypercalcaemia due to VDT ranged from 4 months to 18 months (median–7 months).[6]
In the presence of active infection, dysregulation of vitamin D metabolism occurs.[14] In contrast to the renal 1,25(OH)2 vitamin D production which is tightly regulated, the extrarenal production of 1,25(OH)2 vitamin D is regulated by cytokines, lipopolysaccharide, nitric oxide and intracellular vitamin D binding protein which stimulate the conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D by activating the enzyme CYP27B1.[15] Since the extrarenal conversion is dependent on the supply of 25(OH) vitamin D, the external supply of vitamin D in the presence of intracellular infection can cause hypervitaminosis D.[14] This could explain the mechanism of VDT in our patient where intramuscular vitamin D injection acted as substrate and increased conversion of 25(OH) vitamin D to 1,25(OH)2 vitamin D occurred due to the presence of chronic infection (gluteal abscess).
Bisphosphonates have been used to mitigate the hypercalcaemia due to hypervitaminosis D.[71617] Apart from increasing absorption of calcium from GI tract, 1,25(OH)2 vitamin D also stimulates calcium and phosphorus release through bone matrix degradation by inducing the maturation of osteoclasts. Osteoclast activity is mediated by the expression of receptor activator of nuclear factor-κB ligand (RANKL).[16] Denosumab, a monoclonal antibody approved for the treatment of osteoporosis, binds to RANKL and inhibits bone resorption via inhibition of osteoclast activity. In view of renal dysfunction, denosumab was used rather than other bisphosphonates. However, further investigation is needed regarding the use of denosumab in vitamin D-induced hypercalcaemia.
Ketoconazole is an imidazole antifungal that inhibits macrophage 1 α-hydroxylation of 25-hydroxy vitamin D3 and has been used in paraneoplastic hypercalcaemia and in granulomatous diseases where steroids have to be avoided. We could not use ketoconazole as patient was lost to follow-up in between after the steroid trial.
In conclusion, vitamin D intoxication, although uncommon, can occur even in the standard dosage due its overzealous usage. This case highlights the importance of follow-up to confirm the resolution of VDT. High-dose vitamin D supplements given intramuscularly in the presence of active infection/abscess and ongoing weight loss can have exaggerated responses leading to toxicity and, therefore, should be avoided. The importance of a thorough history and examination cannot be overemphasized.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol (Oxf). 2009;70:680-4.
- [Google Scholar]
- Study of bone mineral density in resident doctors working at a teaching hospital. J Postgrad Med. 2010;56:65.
- [Google Scholar]
- Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: Implications for treatment and prophylaxis. J Clin Endocrinol Metab. 2013;98:2709-15.
- [Google Scholar]
- Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: Efficacy and safety data. Med J Aust. 2005;183:10-2.
- [Google Scholar]
- Vitamin D toxicity: A prospective study from a tertiary care centre in Kashmir valley. Indian J Endocrinol Metab. 2019;23:363.
- [Google Scholar]
- Vitamin D intoxication causes hypercalcaemia by increased bone resorption which responds to pamidronate. Clin Endocrinol (Oxf). 1995;43:531-6.
- [Google Scholar]
- Vitamin D toxicity–A clinical perspective. Front Endocrinol (Lausanne). 2018;9:550.
- [Google Scholar]
- Deposition in and release of vitamin D3 from body fat: Evidence for a storage site in the rat. J Clin Invest. 1971;50:679-87.
- [Google Scholar]
- Contribution of adipose tissue to plasma 25-hydroxyvitamin D concentrations during weight loss following gastric bypass surgery. Obesity (Silver Spring). 2011;19:588.
- [Google Scholar]
- Vitamin D stored in fat tissue during a 5-year intervention affects serum 25-hydroxyvitamin D levels the following year. J Clin Endocrinol Metab. 2017;102:3731-8.
- [Google Scholar]
- γ-Interferon-induced resistance to 1,25-(OH)2 D3 in human monocytes and macrophages: A mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab. 1997;82:2222-32.
- [Google Scholar]
- Long-term hypervitaminosis D-induced hypercalcaemia treated with glucocorticoids and bisphosphonates. BMJ Case Rep. 2020;13:e233853.
- [Google Scholar]
- Pamidronate treatment in acute vitamin D intoxication. J Endocrinol Invest. 2004;27:680-2.
- [Google Scholar]







