Translate this page into:
Two Decades Outcomes of Posttransplant Immunoglobulin A Nephropathy in Live Donor Renal Transplantation
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The data on long-term outcomes of posttransplant immunoglobulin A nephropathy (IgAN) are confounding and vary with geography and ethnicity worldwide. We aimed to study the long-term graft outcomes of patients with posttransplant IgAN in the northern Indian cohort.
Methods:
The long-term graft outcomes of 51 live donor renal transplant recipients with biopsy-proven posttransplant IgAN (recurrence/de novo) were analyzed. The risk factors for graft failure in the posttransplant IgA groups were analyzed using the Cox regression analysis.
Results:
Out of the total of 51 patients who had posttransplant IgAN, 40 patients had a biopsy-proven native kidney IgAN. The mean duration of the clinical presentation of posttransplant IgAN was 62.4 months (5.2 years) posttransplant. Proteinuria at the time of biopsy was 3.03 ± 2.2 g/day, and 41.2% had proteinuria of more than 3 g/day at the time of biopsy. The estimated 1, 5, 10, and 20 years patient survival was 98%, 95.4%, 75.9%, and 25.2%, respectively, and the estimated 1, 5, 10, and 20 years graft survival was 98%, 88.5%, 44.6%, and 11.9%, respectively, in patients who had posttransplant IgA. Many of the traditional risk factors associated with progression in native kidney IgAN, such as the degree of proteinuria, Oxford MEST (mesangial and endocapillary hypercellularity, segmental sclerosis, and interstitial fibrosis/tubular atrophy) scoring, recipient's age, and sex were not predictive of early graft failure among patients with posttransplant IgAN. In our cohort, the only significant graft failure predictor was serum creatinine at 5 years. Chronic antibody-mediated rejection (ABMR) was seen in 21.6% of patients with posttransplant IgAN. Whether this coexistence of chronic ABMR is an incidental finding or posttransplant IgAN predisposes to chronic ABMR requires further investigation.
Conclusion:
Posttransplant IgAN is associated with poor long-term graft outcomes in live donor renal transplants. Proteinuria and MEST scoring were not predictive of graft failure in living donor posttransplant IgAN.
Keywords
IgA nephropathy
outcomes
recurrence
renal transplantation
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis worldwide.[1] It is slowly progressive and results in end-stage kidney disease (ESKD) in 20% to 40% of patients at around 20 years after its diagnosis.[2] Kidney transplantation (KT) is the ultimate treatment for patients with IgAN and ESKD. Unfortunately, KT is also not always curative, as the histological recurrence without clinical manifestations can occur in up to 53% of cases.[3] The recurrence rates ranged from 13% to 50% in various studies for patients receiving graft biopsies for clinical indications.[4567891011121314151617181920] Recurrence seems to be a time-dependent event, whose prevalence increases with increasing duration of follow-up, resulting in late graft loss.[20] However, the outcome data of recurrence are confounding with varied geography and ethnicity. The available studies comparing the graft survival of IgAN patients undergoing transplant with that of control groups suggest that during the first 5 years after transplantation, allograft survival for primary IgAN patients is better than that of patients with other primary diseases.[4521] At 10 years, the graft survival of IgAN patients becomes comparable with that of other conditions[46]; and it becomes worse after 12 years.[7] Most of the studies showed that patients with clinical recurrence of IgAN have a lower long-term graft survival than patients with no recurrence.[7] Importantly, prognostic factors for early graft failure in these patients remain to be fully elucidated. Previous studies have suggested that transplants from living-related donors,[1315] proteinuria, hypertension, and graft dysfunction at diagnosis were associated with inferior graft survival.[1516] Shreds of evidence also indicate that the Oxford classification can be useful in identifying patients with worse outcomes. The present study was aimed to analyze the long-term graft outcomes of patients with posttransplant IgAN and elucidate the risk factors for graft failure in patients with posttransplant IgAN.
Materials and Methods
This retrospective study investigated all the living donor renal transplant recipients from 1998 to 2018 and identified 51 patients of posttransplant IgAN (proven recurrence, n = 41). All the relevant data were retrieved from the hospital electronic information system, which were prospectively stored in electronic format. The study was approved by the ethics committee of the institute.
Biopsy and histological evaluation
The indication of posttransplant biopsies was acute renal allograft dysfunction with a rise in serum creatinine of more than 30% from the baseline, persistent proteinuria >0.5 g/day, or persistent hematuria of nonurological causes. All biopsies were evaluated by light microscopy, immunofluorescence, and electron microscopy. Diagnosis of IgA was made based on the immunofluorescence finding of dominant or codominant IgA deposition. Diagnosis of rejections was made as per the revised Banff 2017 classification of rejection.
Primary outcomes
The primary outcome of the study was to evaluate the patient survival, graft survival, and death-censored graft survival of the cohort. Graft survival was calculated from the date of transplantation to the date of irreversible graft failure signified by the return of long-term dialysis (or retransplantation) or the date of the last follow-up during the period when the transplant was still functioning or the date of death. The death-censored graft survival was calculated from transplantation to the date of irreversible graft failure signified by the return of long-term dialysis (or retransplantation) or the date of the last follow-up during the period when the transplant was still functioning. In death with a functioning graft, the follow-up period was censored to the date of death.
We also evaluated other outcomes such as serum creatinine at 1 and 5 years, and rates of rejections among patients with posttransplant IgA. Predictors of graft failure among patients with posttransplant IgAN were analyzed.
Statistical analysis
Statistical analysis was performed using SPSS IBM Version 21.0. The continuous variables are expressed as mean ± standard deviation. The categorical values are expressed as percentages. The multivariate Cox regression analysis was used to identify the predictors of graft failure of the patients. Kaplan–Meier survival analysis was used to compare the patient survival with the event as death of the patient. The death-noncensored graft survival was analyzed with the event as the death plus graft failure, and death-censored graft survival curves were analyzed with only graft failure as the event on the Kaplan–Meier survival analysis.
Results
The demographic and clinical characteristics of patients are reported in Table 1. Out of the total of 51 patients who had evidence of posttransplant IgAN, 40 patients had a biopsy-proven pretransplant IgAN. The mean duration for the clinical presentation of posttransplant IgAN was 62.4 ± 48.7 months (5.2 years) posttransplant. The mean proteinuria at the time of biopsy was 3.03 ± 2.17 g/day, and proteinuria of more than 1 g/day was observed in 84.3% of the patients, and 41.2% of these patients had proteinuria of more than 3 g/day.
| Mean±SD | Median (Range) | |
|---|---|---|
| Age, years | 32.14±9.22 | 33 (16-60) |
| Sex (male) | 92.2% (n=4) | |
| ABO compatible | 98% (n=50) | |
| Dialysis Vintage months | 8.63±10.4 | 6 (2-72) |
| HLA Mismatch | 3.49±1.30 | 3 (1-6) |
| Diabetes Mellitus | ||
| Pretransplant | 2% (n=1) | |
| Posttransplant | 17.6% (n=9) | |
| Induction | ||
| Basiliximab | 60.8% (n=31) | |
| ATG | 27.5% (n=14) | |
| No Induction | 11.8% (n=6) | |
| Related Donor | 80.4% (n=41) | |
| Donor Age (years) | 48.29±10.21 | 50 (27-63) |
| Donor GFR (mL/minute) | 66.89±5.10 | 35.5 (30-50) |
| Donor Sex (female) | 70.6% (n=36) | |
| Immunosuppression | ||
| CSA | 21.6% (n=11) | |
| TAC | 78.4% (n=40) | |
| MMF | 94.1% (n=48) | |
| AZA | 5.9% (n=3) | |
| Creatinine at 1 month | 1.14±0.22 | 1.1 (0.65-1.6) |
| Follow-up (years) | 7.01±3.89 | |
| Creatinine at 1 year (mg/dL) | 1.30±0.28 | |
| Creatinine at 5 years (mg/dL) | 2.0±1.78 | |
| Rejection | ||
| Acute ABMR | 7.8% (n=4) | |
| Chronic ABMR | 21.6% (n=11) | |
| ACR | 21.6% (n=11) | |
| Duration of Recurrence (years) | 5.19±4.06 | |
| Proteinuria (g/day) | 3.03±2.17 |
The allograft biopsy findings of the posttransplant IgAN group were analyzed [Table 2], and the most common light microscopy finding was segmental sclerosis, seen in 49% of the patients (n = 25). Mesangial proliferation (mean = 1) was seen in 41.2% (n = 21), and only a small number of patients showed evidence of endocapillary proliferation (5.9%) and crescents (3.9%). Diffuse global glomerulosclerosis was seen in only 9.8% of the patients (n = 5).
| Pretransplant biopsy (n=41) | |
| HAAS staging | |
| IV | 38.8% (n=7) |
| 1V | 61.1% (n=34) |
| MEST score | |
| M1 | 33.3% (n=6) |
| E1 | 5.5% (n=1) |
| S1 | 50% (n=9) |
| T1 | 44.4% (n=8) |
| T2 | 11.1% (n=2) |
| C1 | 22.2% (n=4) |
| C2 | 11.1% (n=2) |
| 0% | |
| Posttransplant biopsy (n=51) | |
| HAAS staging | |
| I | 17.6% (n=9) |
| II | 11.8% (n=6) |
| III | 25.5% (n=18) |
| IV | 35.3% (n=18) |
| V | 9.8% (n=5) |
| MEST score | |
| M1 | 41.2% (n=21) |
| E1 | 5.9 (n=3) |
| S1 | 49% (n=25) |
| T1 | 17.6% (n=9) |
| T2 | 0% (n=0) |
| C1 | 3.9% (n=2) |
| C2 | 0% (n=0) |
| Proteinuria range | |
| <1 g | 15.7% (n=8) |
| 1-3 g | 43.1% (n=22) |
| >3 g | 41.2% (n=21) |
| Treatment | |
| ACE/ARB | 100% (n=51) |
| Omega-3 fatty acids | 21.6% (n=11) |
| High-dose oral steroids | 5.9% (n=3) |
| IV MPS | 3.9% (n=2) |
HAAS =, MEST=mesangial and endocapillary hypercellularity, segmental sclerosis, and interstitial fibrosis/tubular atrophy, ACE=angiotensin-converting enzyme, ARB=angiotensin II receptor blocker, MPS=mucopolysaccharidosis
All patients with biopsy-proven posttransplant IgAN were treated with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) as antiproteinuric drugs. Omega-3 fatty acids were given to 21.6% of patients (n = 11), and intravenous methylprednisolone pulse to two patients. A short course of high-dose oral prednisolone was given to three patients (starting at 1 mg/kg and tapered to the baseline dose over 1 month). The dose of prednisolone was increased to 10 mg per day in all patients.
Outcomes
A total of seven deaths occurred during the follow-up period. The estimated mean patient survival using Kaplan–Meier Analysis was 15.06 (95% confidence interval [CI] 12.05–18.07) years. The estimated 1, 5, 10, 15, and 20 years mean patient survival was 100%, 95.4%, 75.9%, 51%, and 25.2% respectively, among patients with posttransplant IgAN as shown in Figure 1.

- Kaplan–Meier survival analysis showing patient survival
The graft failure occurred in 21 out of 51 patients, seven died and 14 developed graft failure, during the entire follow-up period. The estimated mean graft survival was 10.89 years and the estimated 1, 5, 10, 15, and 20 years graft survival was 100%, 88.5%, 44.6%, 23%, and 11.9% [Figure 2]. The death-censored graft failure was noted in 14 patients, and the median survival was 13.8 (95% CI 10.82–16.92) years. The estimated death-censored graft survival at 1, 5, 10, 15, and 20 years was 100%, 86%, 65%, 46%, and 46%, respectively, in our cohort of study, estimated to be 59% at 10 years and 47.2% at 20 years in the cohort [Figure 3].

- Kaplan–Meier survival analysis showing death-noncensored graft survival
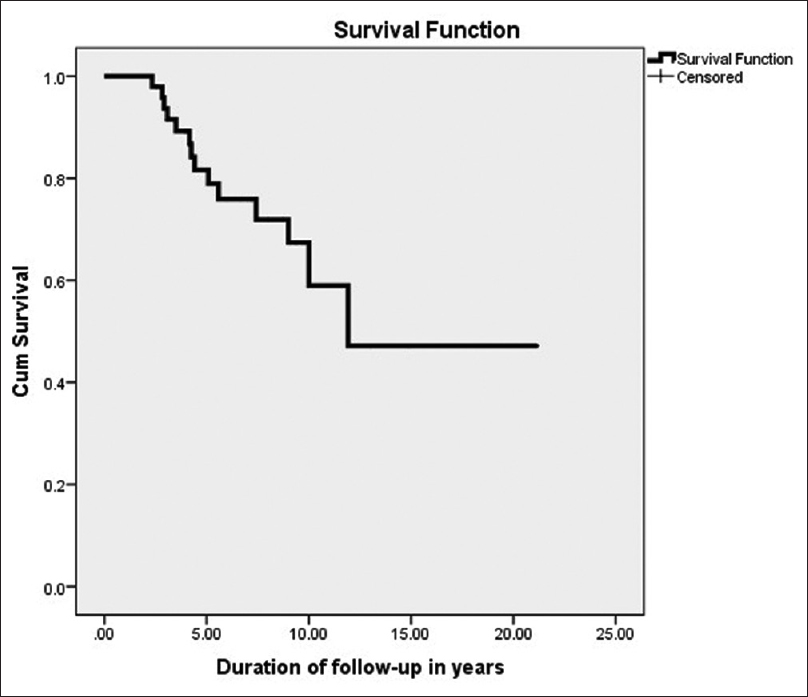
- Kaplan–Meier survival analysis showing death-censored graft survival
The mean serum creatinine at 1-year posttransplant was 1.3 ± 0.28 mg/dL, and the mean serum creatinine value at 5 years was 2.0 ± 1.78 mg/dL. Among these patients with posttransplant IgAN, four patients (7.8%) had acute antibody-mediated rejection (ABMR), and 11 patients (21.6%) had acute T-cell–mediated rejection (TCMR). Biopsy-proven chronic ABMR was seen in a total of 11 patients (21.6%). In the subgroup survival analysis, there was no significant difference in the graft survival or death-censored graft survival among patients who had chronic ABMR and who did not have chronic ABMR in the posttransplant IgAN group as depicted in Figure 4.
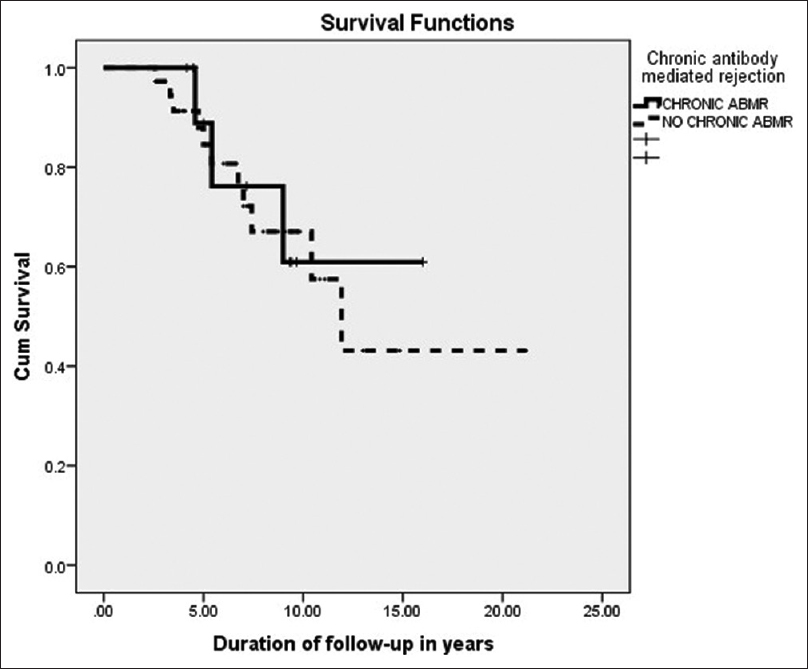
- Kaplan–Meier survival analysis showing death-censored graft survival in posttransplant IgAN with and without chronic antibody-mediated rejection
Risk factors for graft failure in the posttransplant IgA nephropathy group
Using multivariate Cox regression analysis, we tried to identify the risk factors responsible for graft failure in the posttransplant IgAN group, and the results are shown in Table 3. The only significant graft failure predictor was serum creatinine at 5 years (hazard ratio = 2.18, 95% CI 1.34–3.55, P = 0.002). Many of the traditional risk factors associated with progression in native kidney IgAN, such as the degree of proteinuria (P = 0.40), Oxford MEST (mesangial and endocapillary hypercellularity, segmental sclerosis, and interstitial fibrosis/tubular atrophy) scoring, recipient's age (P = 0.67), sex (P = 0.96), and dialysis vintage (P = 0.19) were not associated with graft failure during the long term in these patients with posttransplant IgAN. Donor age (P = 0.78) and donor GFR (P = 0.96) were not a predictors of graft failure.
| Risk factors | Hazard ratio | 95% Confidence interval | P |
|---|---|---|---|
| Recipient age (per year) | 0.98 | 0.94-1.04 | 0.67 |
| Recipient sex (male vs. female) | 0.96 | 0.125-7.36 | 0.96 |
| Dialysis vintage (per year) | 1.04 | 0.97-1.11 | 0.19 |
| Time of recurrence (per year) | 3.95 | 0.81-19.15 | 0.87 |
| Renal biopsy | |||
| Mesangial proliferation | 0.57 | 0.23-1.4 | 0.22 |
| Endocapillary proliferation | 0.55 | 0.73-4.19 | 0.56 |
| Segmental sclerosis | 1.76 | 0.68-4.4 | 0.23 |
| Tubular atrophy | 1.66 | 0.59-4.67 | 0.33 |
| Crescents | 0.97 | 0.12-7.59 | 0.98 |
| HLA mismatch (per mismatch) | 0.87 | 0.30-2.49 | 0.80 |
| Donor age (per year) | 1.00 | 0.96-1.05 | 0.78 |
| Donor sex (male vs. female) | 0.65 | 011-1.98 | 0.43 |
| Donor GFR (per mL/minute) | 0.99 | 0.90-1.10 | 0.96 |
| Proteinuria (per g/day) | 1.09 | 0.88-1.34 | 0.40 |
| Creatinine at 1 year | 4.6 | 0.30-57.69 | 0.23 |
| Creatinine at 5 year | 2.18 | 1.34-3.55 | 0.002 |
| Chronic ABMR | 0.89 | 0.34-2.33 | 0.82 |
| Acute rejections | 4.95 | 0.82-29.93 | 0.08 |
| CMV infection | 1.42 | 0.48-4.19 | 0.51 |
| Induction regimen (ATG vs. Basiliximab) | 0.627 | 0.15-2.55 | 0.51 |
| Tacrolimus vs. Cyclosporine based | 0.421 | 0.94-1.89 | 0.25 |
IgAN=immunoglobulin A nephropathy, HLA=human leukocyte antigens, GFR=glomerular filtration rate, ABMR=antibody-mediated rejection, CMV=cytomegalovirus, ATG=antithymocyte globulin
Discussion
In this study, we have reported the findings of a single-center experience of the long-term outcomes of posttransplant IgAN among live donor renal transplant recipients. If we look at the overall outcomes after live donor KT, the data from the United States show a 5-year patient survival of 93.1% and a 5-year allograft survival of 84.6%.[22] The overall reported survival rates for living donor transplants are higher in Europe, with a patient survival of 94.3% and a graft survival of 86.9% at 5 years.[23] The ANZDATA (Australian and New Zealand Dialysis and Transplant) Registry shows even a higher patient survival of 95% and graft survival of 90% at 5 years.[24] In India, the outcomes of renal allograft vary from center to center. A large government institute from Chandigarh had reported a 5-year patient survival of 83% and a 5-year graft survival of 79% among living donor transplants.[25] Data from a large tertiary care military hospital in North India have revealed an estimated graft survival at 5 years of 80.5%.[26] The available studies comparing the graft survival of posttransplant IgAN patients with overall survival show variable outcomes[10111213141827282930313233343536] as shown in Table 4.
| Author(s) | Year | Recurrence number | Follow-up (mean) | Results (patients with recurrence) |
|---|---|---|---|---|
| Kessler et al.[10] | 1998 | 13 | 68.1±37.2 months | Similar 1-, 5-, and 8-year graft survival and serum creatinine |
| Frohnert et al.[11] | 1997 | 13 | >10 years | 71% graft failure |
| Ohmacht et al.[12] | 1997 | 14 | 51 months | 71% graft failure |
| Matsugami et al.[27] | 1998 | 12 | — | 68.8% graft loss at 10 years |
| Freese et al.[14] | 1999 | 13 | 60 months (median) | 46.1% graft failure at 5 years |
| Kim et al.[4] | 2001 | 19 | — | Similar graft survival at 10 years |
| Wang et al.[15] | 2001 | 14 | 55 months | Graft dysfunction in 29% at 5 years |
| Andresdottir et al.[5] | 2001 | 7 | 5.6±4.5 years | Similar estimated 10-year survival |
| Choy et al.[7] | 2003 | 14 | 100±4.5 months | 35.7% graft failure |
| Jeong et al.[28] | 2004 | 39 | 10-year graft survival of 66.5% (comparable) | |
| Moriyama et al.[29] | 2005 | 13 | 10 years | Graft failure in 38.5% vs. 9.2% |
| Chandrakantan et al.[16] | 2005 | 20 | — | 10-year graft survival of 50% vs. 80% |
| Pazik et al.[30] | 2006 | 27 | 61 months | 6.57 times higher risk of graft failure |
| Han et al.[18] | 2009 | 68 | 10 years | 61% graft survival vs. 85.1% |
| Kamal et al.[31] | 2012 | 25 | 6.6 years | Comparable graft survival |
| Moroni et al.[32] | 2012 | 42 | 113.1 months | Death-censored graft survival of 51.2% vs. 68.3% |
| Lemes-Canuto et al.[33] | 2015 | 128 | — | 58.5% graft survival at 10 years |
| Nijim et al.[34] | 2016 | 23 | — | Mean graft survival 6.5±5.1 years (vs. 10.5±3.5 years) |
| Kim et al.[35] | 2017 | 15 | 82.5 months | 73% graft failure |
| Cordeiro et al.[36] | 2018 | 47 | >6 months after IgAN recurrence | 31.9% graft loss |
| Current study | 2021 | 51 | 84.2 months | 10-year graft survival of 44.6% and death-censored graft survival of 59% |
For patients with IgAN who undergo transplant, studies suggest a better outcome at 5 years[4521]; comparable outcome at 10 years,[46] and poor outcome after 12 years.[7] For posttransplant IgAN, most studies showed that patients with clinical recurrence of IgAN have a lower long-term graft survival than patients without recurrence.[711131618313233343536] Local variations in patient demographics and medical practice can contribute to differences in renal outcomes in patients with IgAN in native kidney disease.[37] These local variations might be important in the posttransplant IgAN as well. To the best of our knowledge, no study from this region has reported posttransplant IgAN outcomes.
Some studies have shown that graft survival is similar in patients with and without recurrence on 10 years follow-up, and IgA recurrence had negligible influence on 5- and 10-year graft survival rates.[531] However, major large and long-term follow-up studies, including ours, showed the influence of IgA recurrence on graft survival. Most previous studies had only a small number of patients with biopsy-proven recurrence of IgAN. Pazik et al.[30] in a study of 27 patients with posttransplant IgAN, found that compared with the control group, patients with IgAN experienced 6.57 times higher risk for dialysis dependence at a follow-up of 61 months. Han et al.[18] also showed that the 10-year graft survival was affected by recurrent IgAN, with 61.0% in the recurrent IgAN group and 85.1% in the nonrecurrent group. An Italian study with perhaps the longest follow-up showed a recurrence rate of 22.1% (n = 42) in their cohort, and the estimated 15-year death-censored graft survival was 68.3% in nonrecurrent group and 51.2% in the recurrent group (P = 0.069).[32] Lemes et al. in a retrospective study with the largest number of patients (n = 146) with posttransplant IgAN[36] found a 10-year patient survival rate of 93.5% in the recurrence group and 100% in the nonrecurrence group. However, the graft survival rates in their study were 58.5% in the recurrent group and 87.2% in the nonrecurrent group. All the above studies have shown that the recurrence of IgAN portends a poor prognosis in terms of graft survival. We, in this study, also found an inferior long-term graft survival among patients who had posttransplant IgAN.
Most of the previous studies have analyzed the risk factors for recurrence of IgAN posttransplant. Only a few have looked into the predictors of early graft failure in patients with posttransplant IgAN. So, the reported data are limited regarding the predictors of early graft failure in IgAN recurrence. Still, it is generally considered that increased urinary protein excretion and increased sclerosis and fibrosis by graft histopathology are associated with an enhanced risk of progressive disease. Kimata et al.[38] showed that 24-hour urine protein excretion ≥1 g and prominent glomerulosclerosis involving 30% of the glomeruli or more was associated with graft loss within 6 years after KT. In contrast, 24-hour proteinuria <1 g combined with glomerulosclerosis in 10% of glomeruli or less was associated with stable allograft function 9 years post KT. Persistent microscopic hematuria, on the other hand, is often the earliest marker of recurrent IgAN, but it does not predict a poor outcome.[3839] In evaluating the predictors of early graft failure in the posttransplant IgAN group, we found contrasting findings to that of the previous studies. The only significant predictor of early graft failure in our study was serum creatinine at 5 years. The degree of proteinuria was not predictive of early graft failure in our study, and neither was Oxford MEST scoring predictive of early graft failure, but this finding could be due to a smaller sample size of our study.
Another finding of our study was that a total of 11 patients (21.6%) in the posttransplant IgAN group had chronic ABMR. Whether this coexistence of chronic ABMR is an incidental finding or posttransplant IgAN predisposes to chronic ABMR requires further investigation. However, in the subgroup survival analysis, there was no significant difference in graft survival or death-censored graft survival among patients who had chronic ABMR with IgAN and who had only IgAN. Our findings are similar to a previous Chinese study by Li et al.[40] who studied the clinicopathological features and prognosis of patients of IgAN superimposed on transplant glomerulopathy (TG). They reported that in a cohort of 49 patients, TG with IgAN patients' prognosis was not significantly different from those without IgAN.
Limitations of the study
Our study's limitations remain its retrospective nature and the relatively fewer number of patients. The lack of a comparative arm is also a drawback. We have not analyzed the risk factors for predicting recurrence.
Conclusion
Our result suggests that graft survival and death-censored graft survival of posttransplant IgAN after living donor renal transplant seems to be worse than the overall graft survival reported in most of the registry data. Our data also show that proteinuria and MEST scoring were not predictive of graft failure in posttransplant IgAN, and creatinine at 5 years posttransplant was predictive of graft failure. Future studies are needed to validate the finding of chronic ABMR coexisting with posttransplant IgA nephropathy and to delineate the risk factors responsible for early graft loss in these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge the renal histopathology laboratory's technicians for their support and assistance in completing the project.
References
- The commonest glomerulonephritis in the world: GA nephropathy. Q J Med. 1987;64:709-27.
- [Google Scholar]
- Recurrence of mesangial IgA nephropathy after renal transplantation. Contrib Nephrol. 1984;40:195-7.
- [Google Scholar]
- Live donor renal allograft in end-stage renal failure patients from immunoglobulin A nephropathy. Transplantation. 2001;71:233-8.
- [Google Scholar]
- Favourable outcome of renal transplantation in patients with IgA nephropathy. Clin Nephrol. 2001;56:279-88.
- [Google Scholar]
- Kidney transplantation in patients with IgA mesangial glomerulonephritis. Kidney Int. 2001;60:1948-54.
- [Google Scholar]
- Renal transplantation in patients with primary immunoglobulin A nephropathy. Nephrol Dial Transplant. 2003;18:2399-404.
- [Google Scholar]
- The clinical course of IgAnephropathy and Henoch-Schönlein purpura following renal transplantation. Transplantation. 1986;42:511-5.
- [Google Scholar]
- Recurrence of IgA deposits/disease in grafts.An Australian registry survey 1980–1990. Contrib Nephrol. 1995;111:13.
- [Google Scholar]
- Recurrence of immunoglobulin A nephropathy after renal transplantation in the cyclosporine era. Am J Kidney Dis. 1996;28:99-104.
- [Google Scholar]
- The fate of renal transplants in patients with IgA nephropathy. Clin Transplant. 1997;11:127-33.
- [Google Scholar]
- Recurrent immunoglobulin A nephropathy after renal transplantation: A significant contributor to graft loss. Transplantation. 1997;64:1493-6.
- [Google Scholar]
- Single-centre long-term results of renal transplantation for IgA nephropathy. Transplantation. 1998;65:1053-60.
- [Google Scholar]
- Clinical risk factors for recurrence of IgA nephropathy. Clin Transplant. 1999;13:313-7.
- [Google Scholar]
- Recurrent IgA nephropathy in renal transplant allografts. Am J Kidney Dis. 2001;38:588-96.
- [Google Scholar]
- Recurrent IgA nephropathy after renal transplantation despite immunosuppressive regimens with mycophenolate mofetil. Nephrol Dial Transplant. 2005;20:1214-21.
- [Google Scholar]
- Antithymocyte globulin (ATG) induction therapy and disease recurrence in renal transplant recipients with primary IgA nephropathy. Transplantation. 2008;85:1505-7.
- [Google Scholar]
- Impact of recurrent disease and chronic allograft nephropathy on the long-term allograft outcome in patients with IgA nephropathy. Transpl Int. 2010;23:169-75.
- [Google Scholar]
- Recurrence of IgA nephropathy among renal allograft recipients from living donors is greater among those with zero HLA mismatches. Transplantation. 2006;82:759-62.
- [Google Scholar]
- Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347:103-9.
- [Google Scholar]
- Outcomes of renal transplantation in patients with immunoglobulin A nephropathy in India. In: J Postgrad Med. Vol 53. Mumbai, India: Medknow Publications Pvt. Ltd; 2007. p. :92-95.
- [Google Scholar]
- Registry of the European Renal Association - European Dialysis and Transplant Association. 2013 Annual Report. Available from: http://www.era-edta-reg.org
- ANZDATA Registry. 38th Report, Chapter 8: Transplantation. Australia and New Zealand Dialysis and Transplant Registry, Adelaide. Available from: http://www.anzdata.org.au
- Predictors of allograft survival and patient survival in living donor renal transplant recipients. Indian J Transplant. 2017;11:42-8.
- [Google Scholar]
- Renal transplantation – An experience of 500 patients. Med J Armed Forces India. 2007;63:107-11.
- [Google Scholar]
- [A clinopathological study of recurrent IgA nephropathy following renal transplantation] Nippon Jinzo Gakkai Shi. 1998;40:322-8.
- [Google Scholar]
- IgA nephropathy in renal allograft-recurrence and graft dysfunction. Yonsei Med J. 2004;45:1043-8.
- [Google Scholar]
- Latest IgA deposition from donor kidney is the major risk factor for recurrent IgA nephropathy in renal transplantation. Clin Transpl. 2005;19:41-8.
- [Google Scholar]
- IgA nephropathy in kidney allograft recipients-therapeutic perspective. Transplant Proc. 2006;38:112-4.
- [Google Scholar]
- Renal transplantation outcome in selected recipients with IgA nephropathy as native disease: A bicentric study. Ann Transplant. 2012;17:45-51.
- [Google Scholar]
- The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol Dial Transplant. 2013;28:1305-14.
- [Google Scholar]
- Iga nephropathy in patients receiving a renal transplant. J Ren Care. 2015;41:222-30.
- [Google Scholar]
- Recurrent IgA nephropathy after kidney transplantation. Transplant Proc. 2016;48:2689-94.
- [Google Scholar]
- Long-term clinical outcomes of first and second kidney transplantation in patients with Biopsy-Proven IgA nephropathy. Transplant Proc. 2017;49:992-6.
- [Google Scholar]
- Clinical features, treatment and prognostic factors of post-transplant immunoglobulin A nephropathy. Ann Transplant. 2018;23:166-75.
- [Google Scholar]
- Continental variations in IgA nephropathy among Asians. Clin Nephrol. 2008;70:377-84.
- [Google Scholar]
- Correlation between proteinuria and prognosis of transplant IgA nephropathy. Transplant Proc. 1996;28:1537-9.
- [Google Scholar]
- Persistent dipstick hematuria following renal transplantation. Clin Transpl. 2004;18:321-6.
- [Google Scholar]
- Clinicopathologic features and prognosis of patients with IgA nephropathy superimposed on transplant glomerulopathy. Zhonghua Yi Xue Za Zhi. 2019;26(99):889-94.
- [Google Scholar]







