Translate this page into:
Subjective Global Nutritional Assessment [SGNA] in Children on Chronic Dialysis- A Prospective Observational Study
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Nutritional assessment in children undergoing chronic dialysis is challenging as no single objective reference tool is available. There is a need to explore the application of the subjective global nutritional assessment (SGNA) tool in these children. This study assessed the nutritional status of children on chronic dialysis using SGNA, evaluated the utility of SGNA parameters in the longitudinal assessment of nutrition, and compared the SGNA tool with other nutritional measures.
Methods:
Children 2-18 years of age on chronic dialysis for at least 1 month were prospectively studied over a period of 18 months with two follow-up visits at least 3 months apart. Malnutrition was diagnosed by SGNA (well-nourished, moderately, and severely malnourished), mid-arm circumference <5th centile for age and gender, and serum albumin <3.8 g/l at baseline and follow-up.
Results:
In 41 children on dialysis (age: 124.8 ± 32 months), 73% had moderate or severe malnutrition by SGNA. Height for age (P = 0.008), weight for height (P = 0.004), dietary intake (P = 0.025) functional capacity (P = 0.001), loss of subcutaneous fat (P < 0.001), and muscle wasting (P < 0.001) were significantly associated with the presence and severity of malnutrition. SGNA showed a poor agreement with MUAC and serum albumin. On follow-up, there was no significant change in the category of nutritional status (P = 0.63) and no individual SGNA parameter was associated with the presence or severity of malnutrition.
Conclusion:
Two-thirds of the children on chronic dialysis were diagnosed with moderate to severe malnutrition by SGNA, while the majority remained in the same category of nutritional status on follow-up. Only half of the parameters used for assessment were strongly associated with the presence and severity of malnutrition. SGNA showed a poor agreement with objective nutritional measures and was not responsive in identifying a change in the nutritional status on follow-up.
Keywords
Dialysis
malnutrition
pediatric chronic kidney disease
SGNA
Introduction
In pursuit of a uniform and comprehensive approach to the diagnosis of undernutrition in children, illness-related definitions have been proposed. These definitions were based on reduced nutrient intake, increased energy expenditure, nutrient loss, and altered nutrient utilization.[1] Chronic kidney disease (CKD) is known to result in undernutrition due to the combined effect of increased energy expenditure, nutrient loss, altered nutrient utilization, energy-protein imbalance, and inflammation. Chronic uremic milieu can create metabolic stress besides causing nutrition-specific manifestations such as edema, stunting, reduced appetite, and muscle wasting.
The subjective global nutritional assessment tool (SGNA) uses both subjective and objective aspects of medical history and physical examination to identify nutritional status. The Kidney Disease Outcomes Quality Initiative (KDOQI) recommends SGNA as a reliable and valid tool in assessing nutritional status in adults on dialysis.[2] SGNA is also observed to be an independent predictor of mortality in adults on both peritoneal and hemodialysis.[34] In children, the acronym SGNA is used to refer to subjective global nutritional assessment. SGNA incorporates 10 parameters (seven items in medical history and three in physical examination) to screen for undernutrition.[5] Medical history includes domains of anthropometry, dietary intake, gastrointestinal symptoms, functional capacity, and metabolic stress of disease. Physical examination integrates assessment of parameters such as muscle mass, subcutaneous fat, and presence of edema.
This multiparameter tool consists of nutrition-related domains besides anthropometry, but it is not clear if these parameters reflect the complex interplay of factors involved in malnutrition due to CKD. Undernutrition is highly prevalent in Indian children with CKD compared to children with CKD in developed regions of the world.[678] The utility of SGNA and its comparison with other objectives measures of nutrition in this cohort of children is not well studied with limited data on validation of SGNA.[9]
A prospective study was undertaken to (a) assess the nutritional status of children on chronic dialysis using SGNA, (b) evaluate the utility of individual SGNA parameters in identifying malnutrition over time, and (c) compare SGNA with objective nutritional measures. Though the term “malnutrition” ideally implies both undernutrition and overnutrition, for the purpose of this study, based on categories defined by SGNA, the term malnutrition is being considered synonymous with undernutrition.
Methods
This prospective study was undertaken in children 2–18 years of age on dialysis (for at least 1 month) from April 2017 to December 2020 as a part of an ongoing larger study on protein-energy wasting in children with CKD. Children were recruited from the pediatric CKD clinic of a tertiary care hospital. Those with bony deformities (in whom height could not be accurately recorded), sick children, or those admitted in hospital within the last 1 month, and those who did not give consent for venipuncture for the study were excluded. Institutional ethical approval and informed consent from parents of children were obtained.
Body weight was recorded on a digital weighing scale (Tulaman Pvt. Ltd, India) to the nearest 0.05 kg, and the height was measured using a stadiometer (Standard steel, India) to the nearest 0.1 cm. Height for age percentile and mid parental height were calculated using reference charts of the Indian Academy of Pediatrics.[10] MUAC was measured using a non-metal measuring tape to the nearest 0.1 cm and interpreted based on age and gender by using reference charts.[11] Serum albumin (g/l) was estimated by enzymatic and bromocresol purple dye-binding methods, respectively, by using Siemens Dimension RxL.
SGNA was undertaken in all recruited children.[5]
SGNA rating form consists of two major components: nutrition-focused medical history and physical examination. Medical history includes seven parameters: height-for-age, weight-for-height, changes in body weight, adequacy of dietary intake, gastrointestinal symptoms, functional capacity, and metabolic stress of disease. Physical examination incorporates three parameters: loss of subcutaneous fat, muscle wasting, and edema. Physical examination findings specific to subcutaneous fat loss were noted on cheeks, ribs, and buttocks, while findings for muscle wasting were observed over the clavicle, shoulder, scapula, thigh, knee, and calf. Presence of edema was examined over the ankle and sacrum. A single assessor blinded to the biochemical and mid-arm circumference measures of the child performed the assessment. Medical history was obtained from the primary caregiver/parent of the child. Children were followed up over a 16-month period, consisting of two follow-up visits with a minimum interval period of 3 months between visits. SGNA, MUAC, and serum albumin values were recorded at each follow-up visit. SGNA was assessed by comparing its performance with MUAC <5th centile for age and gender and serum albumin <3.8 g/l based on the cut-off used for defining muscle wasting and hypoalbuminemia in protein-energy wasting.[12] As weight and height are parameters included within the SGNA, BMI was not used as a comparative measure for this study.
The approach to performing a SGNA for children is detailed elsewhere.[8] First, information about each of the 10 parameters is obtained using a combination of nutrition-focused physical examination and administering an age-related questionnaire to obtain related history. Subsequently, the parameters are assigned ratings as normal, moderate, or severe as per the ratings in the SGNA form.[5] SGNA being subjective does not depend on a numerical scoring system and the overall nutritional status is rated as well-nourished, moderately malnourished, and severely malnourished by using parameters of physical exam and history in the context of each other. The well-nourished category is assigned if the child has few/no physical signs of malnutrition, no weight loss or growth failure, dietary difficulties, nutrition-related functional impairments, or persistent GI symptoms. The moderately malnourished category includes recent weight loss, reduced dietary intake, mild/no loss of subcutaneous fat or muscle with or without functional impairments, or gastrointestinal symptoms. The severely malnourished category includes physical signs of malnutrition, positive findings in medical history, and usually gastrointestinal and functional impairments. For the longitudinal interpretation of change in status of individual SGNA parameters and categories of nutritional status, the following terms were used: “unchanged,” “improved,” and “deteriorated.”
Statistical analysis: Data were analyzed using IBM SPSS statistics for Windows, Version 24.0 (Armonk, NY: IBM Corp.). All categorical data were summarized using frequency and percentages. To study the association between clinical characteristics and nutritional status, the association of individual parameters with nutritional status, and the association of status of individual parameters with the status of malnutrition over time, Chi-square test or Fisher's exact test was used. SGNA was compared with MUAC and serum albumin by computing kappa agreement statistics. The P value was considered significant at 5% level of significance for all comparisons.
Results
Forty-one children with kidney failure on dialysis were recruited (66% boys, aged 124.8 ± 32 months). The majority (36/41) were on continuous ambulatory peritoneal dialysis (CAPD) while four were on hemodialysis and one received hybrid dialysis (once a week hemodialysis and daily peritoneal dialysis) with a dialysis vintage of 7 (3,18) months. None of the children were on gastrostomy or tube feeds. Dietary and medical management were undertaken as per the standard practice of the treating team. The most common cause for kidney failure was congenital anomalies of the kidney and urinary tract (68%). From the time of recruitment, the median (IQR) duration for 1st follow-up was 4 (4,6) months and 2nd follow-up was 4 (3,10) months. In the overall cohort, 11 (27%) had severe stunting, 19 (48%) had a MUAC of <5th centile, 75.6% had serum albumin <3.8 g/l, and only one child presented with edema.
Malnutrition as determined by SGNA at recruitment: Thirty (73%) children were diagnosed to be malnourished by SGNA [19/30 (63%) with moderate and 11/30 (37%) with severe malnutrition]. Age, gender, dialysis vintage, and etiology of kidney disease were not significantly different in those with and without malnutrition detected by SGNA as depicted in Table 1.
| Parameters | Baseline SGNA rating in CKD 5D | P | |
|---|---|---|---|
| Malnourished (n=30) | Well-nourished (n=11) | ||
| Age (months) mean±SD | 121.8±39.4 | 127.9±54 | 0.69 |
| Gender | |||
| Male | 20 | 7 | 0.85 |
| Female | 10 | 4 | |
| Duration on dialysis months [median IQR] | 9 (5,21) | 5 (2,7) | 0.054 |
| Etiology of CKD (n,%) | |||
| Non glomerular disease | 23 (82.1%) | 5 (17.8%) | 0.057 |
| Glomerular disease | 7 (53.8%) | 6 (26.8%) | |
Performance of 10 parameters of SGNA in the diagnosis and determining severity of malnutrition: In 41 patients at recruitment, height-for-age (P = 0.008), weight-for-height (P = 0.004), dietary intake (P = 0.025) functional capacity (P = 0.001), loss of subcutaneous fat (P < 0.001), and muscle wasting (P < 0.001) were significantly associated with the presence as well as severity of malnutrition. Changes in body weight, gastrointestinal symptoms, metabolic stress, and edema were associated with neither presence nor severity of malnutrition [Table 2].
| SGNA parameters | Well-nourished (n,%) n=11 | Moderate malnutrition (n,%) n=19 | Severe malnutrition (n,%) n=11 | P |
|---|---|---|---|---|
| Height | ||||
| Normal | 9 (56) | 6 (38) | 1 (6) | 0.008 |
| Moderately less | 2 (14) | 7 (50) | 5 (36) | |
| Severely less | 0 | 6 (55) | 5 (45) | |
| Weight | ||||
| Normal | 4 (57) | 3 (43) | 0 | 0.004 |
| Moderately less | 7 (50) | 4 (29) | 3 (21) | |
| Severely less | 0 | 12 (60) | 8 (40) | |
| Change in body weight | ||||
| No change | 8 (33) | 12 (50) | 4 (17) | 0.191 |
| Improved | 3 (18) | 7 (41) | 7 (41) | |
| Worsened | 0 | 0 | 0 | |
| Diet intake | ||||
| Adequate | 9 (39) | 1252) | 2 (9) | 0.025 |
| Hypocaloric | 2 (12) | 7 (41) | 8 (47) | |
| Starvation | 0 | 0 | 1 (100) | |
| GI symptoms | ||||
| None | 11 (28) | 17 (44) | 11 (28) | 0.29 |
| Not daily | 0 | 2 (100) | 0 | |
| Daily | 0 | 0 | 0 | |
| Functional capacity | ||||
| Energetic | 10 (63) | 4 (25) | 2 (13) | 0.001 |
| Restricted | 1 (4) | 15 (63) | 8 (33) | |
| Little or no play | 0 | 0 | 1 (100) | |
| Metabolic stress | ||||
| None | 3 (60) | 2 (40) | 0 | 0.14 |
| Moderate | 8 (22) | 17 (47) | 11 (31) | |
| Severe | 0 | 0 | 0 | |
| Loss of subcutaneous fat | ||||
| Normal | 11 (58) | 7 (37) | 1 (5) | <0.001 |
| Moderate | 0 | 9 (69) | 4 (31) | |
| Severe | 0 | 3 (33) | 6 (67) | |
| Muscle Wasting | ||||
| None | 8 (62) | 4 (31) | 1 (8) | <0.001 |
| Moderate | 3 (17) | 12 (67) | 3 (17) | |
| Severe | 0 | 3 (30) | 7 (70) | |
| Oedema | ||||
| Normal | 4 (31) | 7 (54) | 2 (15) | 0.44 |
| Moderate | 7 (26) | 12 (44) | 8 (30) | |
| Severe | 0 | 0 | 1 (100) |
Comparison of SGNA with MUAC and serum albumin at recruitment and follow up: At recruitment (n = 41) and 1st follow-up (n = 38), low MUAC was significantly associated with the presence and severity of malnutrition detected by SGNA. Among those with MUAC <5th centile (n = 19), 5.2% were well-nourished, 47.4% had moderate malnutrition, and 47.4% had severe malnutrition. In those with MUAC >5th centile, 42.9% were well-nourished, 47.6% had moderate malnutrition, and 9.5% had severe malnutrition (P = 0.004).
In the first follow-up, among those with MUAC <5th centile (n = 14), 7.1% were well-nourished, 50% had moderate malnutrition, and 42.9% had severe malnutrition. In those with MUAC >5th centile, 43.5% were well-nourished, 52.1% had moderate malnutrition, and 4.4% had severe malnutrition (P = 0.005). The same association was not evident at 2nd follow-up (n = 30, P = 0.20). The level of agreement between SGNA and MUAC for diagnosis of malnutrition was poor (k = 0.36). In addition, presence or severity of malnutrition was not associated with hypoalbuminemia at recruitment and follow-up (P > 0.05) with a poor level of agreement (k = 0.16).
Longitudinal assessment of nutritional status by SGNA: Among 41 children, only 29 completed both follow-up visits [3 expired, 2 received kidney transplantation, and 7 were lost to follow-up]. Among those who followed up, 9/29 (31%), 7/29 (24%), and 6/29 (21%) had no malnutrition at baseline, 1st, and 2nd follow-up, respectively. The proportion of children with malnutrition (moderate and severe categories combined) increased during follow-up [recruitment: 20/29 (69%), 1st follow-up [22/29 (76%)], and 2nd follow-up [23/29 (79%)] but did not reach a statistical significance [Figure 1]. Worsening of body weight, starvation, daily gastrointestinal symptoms, severe reduced functional capacity, moderate to severe metabolic stress, and severe edema were parameters that were noted to be least frequently fulfilled across the three time points [Table 3].
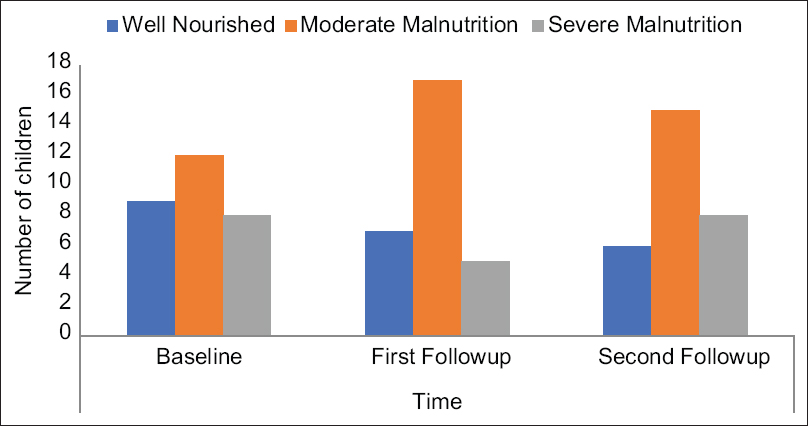
- SGNA categories of nutritional status at baseline and follow-up (n = 29)
| SGNA parameters (n,%) | Baseline (n=41) | 1st follow-up (n=38) | 2nd follow-up (n=30) |
|---|---|---|---|
| Height for age | |||
| Normal | 16 (39.0) | 15 (39.4) | 10 (33.3) |
| Moderately less | 14 (34.1) | 13 (34.2) | 13 (43.3) |
| Severely less | 11 (26.8) | 10 (26.3) | 7 (23.3) |
| Weight for age | |||
| Normal | 7 (17.0) | 10 (26.3) | 5 (16.6) |
| Moderately less | 14 (34.1) | 13 (34.2) | 14 (46.6) |
| Severely less | 20 (48.7) | 15 (39.4) | 11 (36.6) |
| Change in body weight | |||
| No change | 24 (58.5) | 23 (60.5) | 20 (64.5) |
| Improved | 17 (41.4) | 15 (39.4) | 11 (35.4) |
| Worsened | 0 | 0 | 0 |
| Diet intake | |||
| Adequate | 23 (56.1) | 22 (57.8) | 18 (60) |
| Hypocaloric | 17 (41.4) | 16 (42.1) | 12 (40) |
| Starvation | 1 (2.4) | 0 | 0 |
| GI symptoms | |||
| None | 39 (95.1) | 37 (97.3) | 29 (96.6) |
| Not daily | 2 (4.8) | 1 (2.6) | 1 (3.3) |
| Daily | 0 | 0 | 0 |
| Functional capacity | |||
| Energetic | 16 (39.0) | 12 (31.5) | 15 (50) |
| Restricted | 24 (58.5) | 25 (65.7) | 14 (46.6) |
| Little or no play | 1 (2.4) | 1 (2.6) | 1 (3.3) |
| Metabolic stress | |||
| None | 5 (12.2) | 7 (18.4) | 5 (16.6) |
| Moderate | 36 (87.8) | 30 (78.9) | 25 (83.3) |
| Severe | 0 | 1 (2.6) | 0 |
| Loss of subcutaneous fat | |||
| Normal | 19 (46.3) | 17 (44.7) | 11 (36.6) |
| Moderate | 13 (31.7) | 12 (31.5) | 12 (40) |
| Severe | 9 (21.9) | 9 (23.6) | 7 (23.3) |
| Muscle Wasting | |||
| None | 13 (31.7) | 12 (31.5) | 5 (16.6) |
| Moderate | 18 (43.9) | 14 (36.8) | 15 (50) |
| Severe | 10 (24.3) | 12 (31.5) | 10 (33.3) |
| Oedema | |||
| Normal | 13 (31.7) | 10 (26.3) | 6 (20) |
| Moderate | 27 (65.8) | 28 (73.6) | 23 (76.6) |
| Severe | 1 (2.4) | 0 | 1 (3.3) |
On follow-up, change in the status of individual SGNA parameters and the categories of malnutrition were further grouped as “unchanged,” “improved,” and “deteriorated.”
The status of individual SGNA parameters (height-for-age, weight-for-height, changes in body weight, gastrointestinal symptoms, functional capacity, metabolic stress, subcutaneous fat loss, muscle wasting, and edema) on follow-up were not associated with a change in the nutritional status (P > 0.05). Only dietary intake was significantly associated with the categories of malnutrition on follow-up (P = 0.042).
Discussion
Children on dialysis are vulnerable to malnutrition, necessitating early recognition for appropriate interventions to be implemented. Malnutrition was present in the majority (73%) of children on chronic dialysis using SGNA. Of the ten parameters, only five (anthropometry, diet intake, functional capacity, subcutaneous fat loss, and muscle wasting) were noted to be strongly associated with the presence and severity of malnutrition. SGNA showed a poor agreement with objective measures such as MUAC and serum albumin. Among those who completed follow-up (median: 8 months), no significant change in the category of nutritional status was observed and the profile of individual parameters on follow-up had no impact on the overall nutritional status.
The International Pediatric Peritoneal Dialysis Network reported the prevalence of undernutrition (based on BMI z scores) to be 8.9% in 1001 children on chronic peritoneal dialysis across the globe, with a higher burden (20%) observed in South Asia.[13] In the presence of edema and fluid overload, objective assessment tools for the diagnosis of malnutrition in children with CKD may be unreliable and there is no single reference tool available to date.[1415] Moreover, BMI as a measure fails to differentiate muscle wasting and subcutaneous fat loss. This being also true for adults with CKD has led to the exploration of a combination of objective and subjective assessments. It is a paradox that assessments such as SGNA that include subjective parameters have been found to be more robust in predicting outcomes of adults on dialysis compared to only objective measures.[16] The burden of malnutrition detected by SGNA is reported to be higher than that detected by objective anthropometry measures in children without CKD.[1718] Similarly, in children on dialysis, we noted that SGNA detected malnutrition in 73% compared to 48% by MUAC.
Clinical judgment based on physical examination has always been the cornerstone of nutritional assessment in children. The Pediatric Renal Nutrition Taskforce guidelines on nutritional assessment for children with CKD, including those on dialysis, highlights the importance of nutrition-focused physical examination.[15] The SGNA importantly considers nutrition-focused physical examination besides anthropometry and functional parameters. Assessment for the entity “protein-energy wasting” (PEW) also includes a combination of objective (anthropometry and biochemistry) and subjective (appetite) parameters.[12] We recently reported the prevalence of PEW in children on chronic dialysis to be 74% in those on peritoneal dialysis and 100% undergoing hemodialysis.[19] Among parameters used to diagnose PEW, anthropometry measures and appetite were the only useful parameters while biochemical measures were not associated with PEW. This further supports the need to explore the utility of SGNA, which does not incorporate biochemical measures but includes anthropometry, diet, functional parameters, and physical examination.
In our study, five parameters, namely height-for-age, weight-for-height, dietary intake, functional capacity, loss of subcutaneous fat, and muscle wasting, were significantly associated with the presence and severity of malnutrition. In contrast, we did not observe a change in body weight, gastrointestinal symptoms, metabolic stress, and edema to be associated with the presence or severity of malnutrition. Very few studies have examined the impact of individual SGNA parameters on the overall classification of nutritional status in children. In non-CKD children who underwent thoracic or abdominal surgeries, the individual SGNA parameters among young children that most influenced SGNA rating were physical signs of muscle wasting, gastrointestinal symptoms, and metabolic stress. In older children, physical evidence of fat wasting, serial weight loss, gastrointestinal symptoms, and stunting impacted the SGNA rating.[20]
Edema is a relevant confounding parameter for nutritional assessment in CKD. It was observed that moderate edema was noted in the majority and only one child presented with severe edema. Despite this, severe loss of subcutaneous fat and muscle wasting were observed in about one-fifth to one-fourth of the cohort, respectively. In our cohort, functional capacity was found to be useful in identifying those with malnutrition and underscores its relevance for the overall wellbeing of the child.
In contrast to adults with CKD, there is very little work undertaken on the comparison of SGNA with objective measures in children. In adults on chronic dialysis, SGNA is a valid diagnostic tool to assess nutritional status and has also proven to be an independent predictor of mortality.[23212223] A study on children with cerebral palsy reported only a fair level of agreement of subjective assessment against objective measures.[17] In another group of children subjected to thoracic or abdominal surgeries, SGNA was found to have a moderate-to-fair correlation with anthropometric measures such as weight, height, BMI, and MUAC.[20] A study in 68 children with CKD noted good association between SGNA and objective measures such as weight, BMI, and MUAC, but not with serum albumin.[9] However, there was no mention made on the level of agreement between tools in these studies. As measures of weight and height are already included under SGNA, for comparison, we considered only measures of MUAC and serum albumin that are not incorporated in the SGNA. Our findings reveal a good association of SGNA with MUAC but not with serum albumin. A high cut-off value of serum albumin used based on the definition of protein energy wasting in this group of children could be a potential reason for a poor association with SGNA. Though a significant association between SGNA and MUAC was observed at baseline and 1st follow-up, the association was not maintained at the 2nd follow-up, which could be due to drop in numbers on follow-up.
With regard to longitudinal tracking of nutritional status in children on dialysis, evidence is limited. Children with undernutrition on peritoneal dialysis from the IPPN data were observed to have BMI increase over a median follow-up period of 15 months. Gastrostomy was strongly associated with an improvement of the undernourished state. However, concerns pertaining to the interpretation of BMI in this group of children were reported as an improvement in fluid overload status was reflected by a loss in weight and therefore a reduced BMI. In our cohort, nutritional status in the majority of children remained within the same category of SGNA rating over a median period of 8 months and none of them were on gastrostomy/tube feeds. SGNA in general is not designed to detect acute changes.[5] It has been proposed that height and weight velocities identified in those detected with malnutrition by SGNA require at least 6 months period to reveal a trend on the growth chart.[24] However, having a follow-up period of a minimum 7 to a maximum of 16 months, a significant change was not observed in our cohort. The interobserver reproducibility of SGNA has been reported to be only fair with discrepancies occurring more between classifications of “normal to moderate” compared to “moderate to severe malnutrition.”[20] Our study had only one assessor who undertook SGNA so we could not assess the interrater variability.
Detecting malnutrition in children with CKD is critical as it has an impact on morbidity, quality of life, and mortality.[68] In a systematic review of validating nutritional assessment tools that comprised 26 studies on hospitalized stable children (those with renal disorders being excluded), SGNA was used as an assessment tool in only three studies.[25] In these studies, SGNA was used to validate other nutritional screening assessments in hospitalized children and in those with cancer.[262728] Being mindful of the fact that SGNA is time-consuming, it is critical to evaluate its utility, relevance, and scope as an assessment tool. This is one of the very few studies evaluating children on dialysis prospectively for nutritional status by SGNA. Being a lead center for pediatric chronic peritoneal dialysis in the region, we had the opportunity to longitudinally monitor children for nutritional status. Nevertheless, this study has notable limitations. First, children with mild malnutrition are not identified with a SGNA. An interrater variability of the assessor was not estimated. The numbers lost to follow-up contributed to a relatively small number of children toward the end of the study.
In conclusion, two-thirds of children on chronic dialysis were diagnosed to have moderate to severe malnutrition by SGNA, while the majority remained in the same category of nutritional status on follow-up. Only half of the parameters used for assessment were strongly associated with the presence and severity of malnutrition. SGNA showed a good association but poor agreement with MUAC and was also not responsive in identifying a change in the nutritional status on follow-up. Interestingly, despite the nonspecific and semi-quantifiable nature, SGNA occupies a niche segment in adult dialysis patients. Further study on the utility of the SGNA in predicting dialysis adequacy, quality of life, and outcomes of children on chronic dialysis would be meaningful.
Acknowledgements
Ms. Rimcy Paul (CKD nurse) and Ms. Sheeba Collins (Dietician), Department of Pediatric Nephrology, St John's Medical College Hospital, Bangalore and Navajbai Sir Ratan Tata Trust, Mumbai, India.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- American Society for Parenteral and Enteral Nutrition Board of Directors.Defining pediatric malnutrition: A paradigm shift toward etiology-related definitions. J Parenter Enteral Nutr. 2013;37:460-81.
- [Google Scholar]
- KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76((3 Suppl 1)):S1-107.
- [Google Scholar]
- Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158-62.
- [Google Scholar]
- The additional benefit of weighted subjective global assessment (SGA) for the predictability of mortality in incident peritoneal dialysis patients: A prospective study. Medicine (Baltimore). 2017;96:e8421.
- [Google Scholar]
- How to perform Subjective Global Nutritional assessment in children. J Acad Nutr Diet. 2012;112:424-31. e6
- [Google Scholar]
- Anthropometric measures and risk of death in children with end- stage renal disease. Am J Kidney Dis. 2000;36:811-9.
- [Google Scholar]
- Chronic Kidney Disease (CKD): An observational study of etiology, severity and burden of comorbidities. Indian J Pediatr. 2017;84:822-5.
- [Google Scholar]
- CKiD (CKD in children) prospective cohort study: A review of current findings. Am J Kidney Dis. 2012;60:1002-11.
- [Google Scholar]
- Validation of Subjective Global (Nutritional) Assessment (SGNA) in children with CKD. J Ren Nutr. 2011;21:207.
- [Google Scholar]
- Revised IAP growth charts for height, weight and body mass index for 5- to 18-year-old Indian children. Indian Pediatr. 2015;52:47-55.
- [Google Scholar]
- New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34:2540-5.
- [Google Scholar]
- Protein energy wasting in children with chronic kidney disease. Pediatr Nephrol. 2014;29:1231-8.
- [Google Scholar]
- International Pediatric Peritoneal Dialysis Network (IPPN) Registry.Global variation of nutritional status in children undergoing chronic peritoneal dialysis: A longitudinal study of the international pediatric peritoneal dialysis network. Sci Rep. 2019;9:4886.
- [Google Scholar]
- Nutrition in children with kidney disease: Pitfalls of popular assessment methods. Perit Dial Int. 2005;25((Suppl 3)):S143-6.
- [Google Scholar]
- Assessment of nutritional status in children with kidney diseases-clinical practice recommendations from the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol. 2021;36:995-1010.
- [Google Scholar]
- Subjective global assessment of nutrition, dialysis quality, and the theory of the scientific method in Nephrology practice. Artif Organs. 2020;44:1021-30.
- [Google Scholar]
- Subjective Global Nutritional Assessment: A reliable screening tool for nutritional assessment in cerebral palsy children. Indian J Pediatr. 2018;85:15-9.
- [Google Scholar]
- Subjective vs objective nutritional assessment study in children: A cross-sectional study in the northwest of Iran. Nutr Res. 2009;29:269-74.
- [Google Scholar]
- Protein energy wasting in children with chronic kidney disease and end-stage kidney disease: An observational study. J Ren Nutr. 2021;31:270-7.
- [Google Scholar]
- Subjective Global Nutritional Assessment for children. Am J Clin Nutr. 2007;85:1083-9.
- [Google Scholar]
- A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391-8.
- [Google Scholar]
- Prevalence and risk of protein-energy wasting assessed by Subjective Global Assessment in older adults with advanced chronic kidney disease: Results from the EQUAL study. J Ren Nutr. 2018;28:165-74.
- [Google Scholar]
- Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061-70.
- [Google Scholar]
- KDOQI clinical practice guideline for nutrition in children with CKD: 2008 update. Am J Kidney Dis. 2009;53((Suppl 2)):S1-124.
- [Google Scholar]
- Screening and assessment tools for early detection of malnutrition in hospitalised children: A systematic review of validation studies. BMJ Open. 2019;9:e025444.
- [Google Scholar]
- Evaluation of the nutrition screening tool for childhood cancer (SCAN) Clin Nutr. 2016;35:219-24.
- [Google Scholar]
- Validity of nutritional screening tools for hospitalized children. J Nutr Metab. 2014;2014:1-6.
- [Google Scholar]
- Simple nutrition screening tool for pediatric inpatients. J Parenter Enteral Nutr. 2016;40:392-8.
- [Google Scholar]







