Translate this page into:
Atypical HUS Triggered by COVID-19: A Case Report
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We hereby present a case of an atypical hemolytic uremic syndrome (aHUS) precipitated by coronavirus disease 2019 (COVID-19). A 26-year-old male was diagnosed with COVID-19 and acute kidney injury. His kidney biopsy was suggestive of thrombotic microangiopathy. Five sessions of plasmapheresis were done but were discontinued in view of nonrecovery of kidney function. He was then referred for a kidney transplant. On genetic analysis, he was found to have mutations in the complement system (CFHR1 and CFHR3), which suggested this was a case of aHUS precipitated by COVID-19. In view of the high risk of recurrence of the primary disease in live-related kidney donor transplantation, he was advised for simultaneous liver and kidney transplants.
Keywords
AKI
atypical HUS
COVID-19
TMA
Introduction
Acute kidney injury (AKI) is a common complication of coronavirus disease 2019 (COVID-19) with the incidence varying from 0.5% to 80% and an increased incidence in hospitalized patients.[1] AKI can be severe in the form of thrombotic microangiopathy and severe acute tubular necrosis. Most commonly, kidney injury occurs due to hemodynamic abnormality in severe COVID; however, direct invasion and injury by the virus, cytokine storm, abnormal coagulation causing thrombotic injury and necrosis have also been reported.[2] Endothelial damage seen in cases of COVID-19 is one of the important factors associated with the thrombotic state, microvascular damage, and necrosis.[3] Endothelial damage in these patients can also be secondary to complement dysfunction such as atypical hemolytic uremic syndrome precipitated by COVID-19.[4] Atypical hemolytic uremic syndrome (aHUS) is associated with complement system dysregulation either due to mutations in the complement system or because of autoantibodies causing endothelial damage, intravascular thrombosis, and tissue injury. It can be precipitated by pregnancy, infections, or malignancy.[5] We hereby report a case of aHUS precipitated by COVID-19.
Case Report
A 26-year-old male was admitted with fever, myalgia, and sore throat. The reverse transcription polymerase chain reaction (RT-PCR) test from a nasopharyngeal swab confirmed COVID-19 infection. Clinically, he had mild COVID without any need for supplemental oxygen. His lab parameters included normal creatinine with mildly elevated inflammatory markers [Table 1]. He was managed conservatively with antipyretics and multivitamins. From the seventh day of illness, he started complaining of decreased urine output. His creatinine was found to be increased (4 mg/dL). Urine microscopy was suggestive of 5 to 6 red blood cells per high-power field (RBCs/HPF), 2+ protein, and RBC casts. His complete blood count was suggestive of bicytopenia (anemia and thrombocytopenia) [Table 1]. Peripheral smear showed normocytic normochromic anemia and reduced platelets numbers with schistocyte index greater than 4. Ultrasound abdomen showed normal-sized kidneys. Six units of platelets were transfused before kidney biopsy in view of thrombocytopenia. Biopsy was suggestive of acute tubular necrosis with thrombotic microangiopathy (TMA) [Figure 1]. He was initiated on hemodialysis in view of persistent oliguria. His hemoglobin and platelet counts were stabilized after five sessions of plasma exchange; however, he remained oliguric. Genetic testing was performed, which revealed homozygous deletion of the region encompassing upstream region, Exons 1, 2, 3, 6 and Intron 4 of CFHR3 and Intron 1, 3 and Exons 5 and 6 of CFHR1 genes. Anticomplement factor H antibody was negative. Thus, he was diagnosed as a case of aHUS secondary to mutations in CFHR1 and CFHR3. Eculizumab is not available in India and could not be arranged due to financial constraints and he is currently waitlisted for simultaneous liver–kidney transplantation.
| Investigation | At admission | After 7 days | After 2 months |
|---|---|---|---|
| Hemoglobin (g/dL) | 13 | 7 | 9.2 |
| Total leukocyte count (cells per microliter) | 3,400 | 6,000 | 5,400 |
| Platelets (×103 cells per microliter) | 288 | 88 | 175 |
| Total bilirubin (mg/dL) | 0.8 | 2.2 | 1.0 |
| Direct bilirubin (mg/dL) | 0.3 | 0.6 | 0.3 |
| SGOT (U/L) | 21 | 14 | 12 |
| SGPT (U/L) | 14 | 39 | 18 |
| ALP (U/L) | 56 | 76 | 54 |
| LDH (U/L) | 177 | 680 | 120 |
| Total protein (g/dL) | 6.3 | 6.64 | 6.2 |
| Albumin (g/dL) | 3.2 | 2.27 | 2.8 |
| INR | 0.9 | 1.2 | 0.9 |
| Creatinine (mg/dL) | 0.9 | 4.0 | 5.8 |
| Serum HCO3− (mEq/L) | 23 | 18 | 20 |
| C3 (mg/L; 970-1,576) | — | 1,304 | — |
| C4 (mg/L; 162-445) | — | 178 | — |
| CRP, mg/dL (<6) | 17 | 14 | 5 |
| Ferritin, ng/mL (4.63-204) | 758 | 589 | 544 |
| D-dimer, µg/mL (<0.25) | 0.2 | 0.5 | 0.3 |
| IL 6, pg/mL (<6.40) | 14 | 16 | 2 |
| Viral serology: | |||
| Anti-HAV | Negative | ||
| Anti-HEV | Negative | ||
| HBsAg | Negative | ||
| Anti-HCV | Negative | ||
| ANA | Negative | — | |
| ANCA | Negative | — | |
| Urine routine microscopy | pH 6.7 | pH 6.5 | |
| RBCs/HPF 5-6 | RBCs/HPF 2-3 | ||
| Protein 2+RBC casts (+) | WBCs 3-4 | ||
| Protein 1+ Glucose negative | |||
| 24 hours | |||
| Urine protein (g/dL) | 0.8 | 0.7 |
SGOT=serum glutamic oxaloacetic transaminase; SGPT=serum glutamic pyruvic transaminase; ALP=alkaline phosphatase; LDH=lactate dehydrogenase; INR=international normalized ratio; HCO3−=bicarbonate; CRP=C-reactive protein; IL=interleukin; HAV=hepatitis A virus; HEV=hepatitis E virus, HBsAg=hepatitis B surface antigen; HCV=hepatitis C virus; ANA=antinuclear antibody; ANCA=antineutrophil cytoplasmic antibody; RBC=red blood cell; HPF=high-power field; WBC=white blood cell
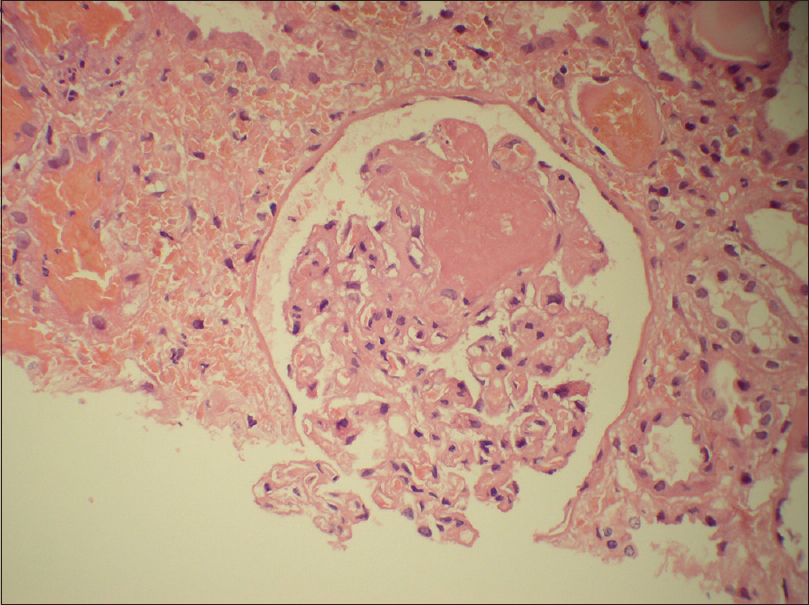
- Photomicrograph showing a glomerulus with fibrin thrombi and mesangiolysis (hematoxylin and eosin stain, original magnification 200×)
Discussion
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause diseases ranging from mild upper respiratory tract infection to critical illness-causing multiorgan damage.
Incidence of AKI varies from 0.5% to 80% with increased incidence in hospitalized patients.[1] The etiology of AKI is multifactorial. Prerenal causes include hypovolemia and sepsis, whereas renal causes include direct viral injury, endothelial damage, complement activation, cytokine storm, and hypercoagulation. All these factors can result in various histological features such as acute tubular necrosis, collapsing glomerulopathy, or thrombotic microangiopathy.[4]
Acute tubular injury is the most common cause of AKI in patients with COVID-19 disease.[6] It accounts for more than 60% of cases of AKI from a study in the United States.[7]
However, COVID-19 can affect all compartments of kidney parenchyma, including glomerulus, tubules, and vessels. Glomerular diseases consist of collapsing glomerulopathy, crescentic glomerulonephritis, thrombotic microangiopathy and podocytopathies. Among glomerular disease, collapsing glomerulopathy is the most common type seen mostly in patients with high-risk APOL1 genotypes.[1]
The incidence of TMA is very rare and was found to be around 2.12% in a study done by Ferlicot et al.[6] while analyzing kidney biopsies of patients having COVID with AKI. Endothelial dysfunction with subsequent microvascular injury-causing thrombotic microangiopathy is an important mechanism of organ failure, including kidneys in COVID-19 patients. SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) receptor to infect the host. ACE2 receptors are expressed in the lung, heart, kidney, and intestines. They are also expressed on endothelial cells. On binding to ACE2 receptors, SARS-CoV-2 causes endothelitis with subsequent vessel injury and organ damage.[8] Patients with inherited defects such as polymorphisms in ACE2 gene,[9] APOL1 genotype,[10] or complement dysfunction are at higher risk for AKI. Few case reports are available in the literature showing the association of TMA with COVID-19. Kulkarni et al.[11] reported a case of TMA secondary to COVID-19 in the background of IgA (immunoglobulin A) nephropathy with no renal recovery. TMA secondary to COVID-19 was also reported by Jhaveri et al.,[12] where the patient ultimately expired.
aHUS is an inherited disorder characterized by a defect in the complement system causing alternate complement pathway activation, endothelial damage, and thrombotic microangiopathy.[13] Complement system mutations associated with HUS include loss of function mutations in complement factor H (CFH; most common), complement factor H–related (CFHR) 1–3, membrane cofactor protein (MCP; most benign among all), complement factor I (CFI), and gain of function mutations in complement factor B (CFB) and C3. CFHR 1–3 mutations are generally associated with autoantibody (anti-factor H) causing TMA, thus making them responsive to plasmapheresis.[14] Our patient had CHFR 1–3 mutation without autoantibody against factor H, explaining the probable reason for poor response to plasma exchange.
aHUS can be triggered by various factors such as upper respiratory tract infections and pregnancy.[5] In our case, COVID-19 was the triggering factor. A similar case report was published by Ville et al.,[15] which reported relapse of aHUS after contracting COVID-19 infection. Also, Mat et al.[16] described a case of aHUS associated with COVID-19 with C3 gene mutation. SARS-CoV-2 may precipitate aHUS by causing endothelial dysfunction that stimulates complement activation causing tissue injury. This complement activation may be amplified in patients with an inherited defect in complement regulation.[15]
Treatment of aHUS consists of plasma exchange with fresh frozen plasma and terminal complement blockades such as eculizumab or liver transplant.[17] Patients with aHUS have a variable rate of recurrence post kidney transplant depending on the mutation. The outcome of kidney transplants with mutations in CFHR1 and CFHR3 is not known. However, except in patients with low risk of recurrence (isolated MCP mutation or those with anti-CFH antibodies that are cleared from blood), prophylactic measures such as eculizumab are used along with a kidney transplant to prevent recurrence.[18] In the case series reported by Alasfar and Alachkar,[19] the authors proceeded with kidney transplant with eculizumab prophylaxis in a patient with a mutation in CFHR1 and CFHR3 to prevent recurrence.
In conclusion, aHUS can be triggered in susceptible individuals even in mild cases of COVID-19 infection.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis. 2020;27:365-76.
- [Google Scholar]
- Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219-27.
- [Google Scholar]
- The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98:314-22.
- [Google Scholar]
- COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16:747-64.
- [Google Scholar]
- AP-HP/Universities/Inserm COVID-19 research collaboration.The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria. Nephrol Dial Transplant 2021:gfab042.
- [Google Scholar]
- The host's angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin Chim Acta. 2020;505:192-3.
- [Google Scholar]
- COVAN is the new HIVAN: The re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol. 2020;16:565-7.
- [Google Scholar]
- Thrombotic microangiopathy causing acute kidney injury in a COVID-19 patient. In: Indian J Nephrol. Vol 4. Mumbai, India: Medknow Publications Pvt. Ltd; 2021. p. :11-5.
- [Google Scholar]
- Atypical hemolytic uremic syndrome. Hematology Am Soc Hematol Educ Program. 2016;2016:217-25.
- [Google Scholar]
- Atypical haemolytic-uraemic syndrome due to heterozygous mutations of CFH/CFHR1-3 and complement factor H 479. Blood Transfus. 2014;12:111-3.
- [Google Scholar]
- Kidney thrombotic microangiopathy after COVID-19 associated with C3 gene mutation. Kidney Int Rep. 2021;6:1732-7.
- [Google Scholar]
- An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15-39.
- [Google Scholar]
- Characteristics, management and outcomes of atypical haemolytic uraemic syndrome in kidney transplant patients: A retrospective national study. Clin Kidney J. 2021;14:1173-80.
- [Google Scholar]
- Atypical hemolytic uremic syndrome post-kidney transplantation: Two case reports and review of the literature. Front Med (Lausanne). 2014;1:52.
- [Google Scholar]







