Translate this page into:
Impact of Kidney Donation on Pregnancy Outcomes: A Retrospective Analysis
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Recent data suggest a risk of gestational hypertension, proteinuria and pre-eclampsia among pregnancies after kidney donation.
Methods:
This retrospective study among females who donated kidneys (1997–2017) at a tertiary renal transplant center in Northern India assessed the maternal and fetal outcomes of their pregnancy. Data of participants were collected using pre-tested semi structured questionnaire.
Results:
In total, 925 female kidney donors (1332 pregnancies) in the pre-donation group and 45 females (48 pregnancies) in the post donation period were included. The mean age of first pregnancy, weight (kg) gain, proportion of history of pre-natal check-up, institutional delivery, and history of unrelated donation was statically significant among the post-donation group. The proportion of pre-eclampsia, gestational hypertension, gestational diabetes, and post-partum hemorrhage was insignificantly higher among the post-donation group with higher preterm birth with low-birth-weight babies. Proteinuria (P < 0.05) was significantly higher among post donation pregnancies. In multivariate analysis, cesarean delivery and low birth weight (<2500 g) were common among the post-donation pregnancy group.
Conclusions:
The study demonstrated no significant risk to maternal outcomes butan increased risk to fetal outcomes in terms of prematurity and low birth weight among the post-donation pregnancy group.
Keywords
Fetal
kidney donation
maternal
outcomes
pregnancy
Introduction
Living kidney donation has transformed the kidney transplant program, halting the need by the recipient on a long waiting list with excellent short- and long-term graft outcomes as compared to deceased donors. This further adds to goodwill among the donors about their contribution to others' lives in a more meaningful way, thus boosting their esteem. Recent evidence suggests a marginal increase in blood pressure and a small absolute reduction of overall kidney functioning following nephrectomy as compared to the general population, reinforcing the need for evolving research on long-term follow-up of living kidney donors.[12] More than 50% of living kidney donors are females, with a significant proportion being in the childbearing age group.[3] It is a known fact that kidney donation as well as pregnancy lead to hyperfiltration, which seems to be a genuine concern.[45] Earlier studies demonstrated that pregnancies after kidney donation were not associated with increased risk of poor maternal and fetal outcomes.[67] Contrary to the above reports, recent studies from Norway revealed a 2.5-fold increase in the adjusted risk of preeclampsia in post-donation pregnancies,[8] while Ibrahim et al.[9] observed that the risk of gestational hypertension, proteinuria, and pre-eclampsia among pregnancies after kidney donation increased with a lower likelihood of full-term delivery.[10] Garg et al.[11] reported these complications to be higher among women older than 32 years as compared to younger donors. Though the data are limited in this regard, women in the reproductive age group who want to donate to their dear ones need to be secured regarding post-donation complications. Thus, we conducted the present study to assess for consequences of pregnancy outcomes among donors in terms of maternal and fetal outcomes.
Study population
In the present retrospective study, we included all women who donated kidneys between 1997 and 2017 at a tertiary renal transplant care center in Northern India. The study was approved by the institute's ethical committee. All female donors were contacted through OPD follow-up as well as telephonically. Written informed consent was taken from the study participants. Data of the study women participants were collected using a pretested semi-structured questionnaire. The women who had at least one pregnancy with at least 20 weeks gestation age were included in the study. Their detailed history and laboratory variables were noted.
In total, 2439 renal transplants were done between 1997 and 2017 (21 years), of which 1758 (72.1%) were female donors. Of these women, 1149 (65.41%) women were successfully contacted and included in our study after obtaining their consent. Among the 1149 responders [mothers: 491 (42.7%), wives: 498 (43.3%), daughters: 24 (2.1%), sisters: 136 (11.9%)], 12.2% (140/1149) females did not have detailed information about their pregnancy and have been excluded from the final analysis. From these women, 5 (0.5%) ladies had pre- and post-donation pregnancies, while 43 (4.3%) had only post-donation pregnancies. Thus, from the remaining 1009 females, 925 females (1332 pregnancies) who had already completed their family were included for the final analysis. Similarly, 45/1009 females (48 pregnancies) who had completed their pregnancy after kidney donation were included and compared with pre-donation pregnancies [Figure 1].
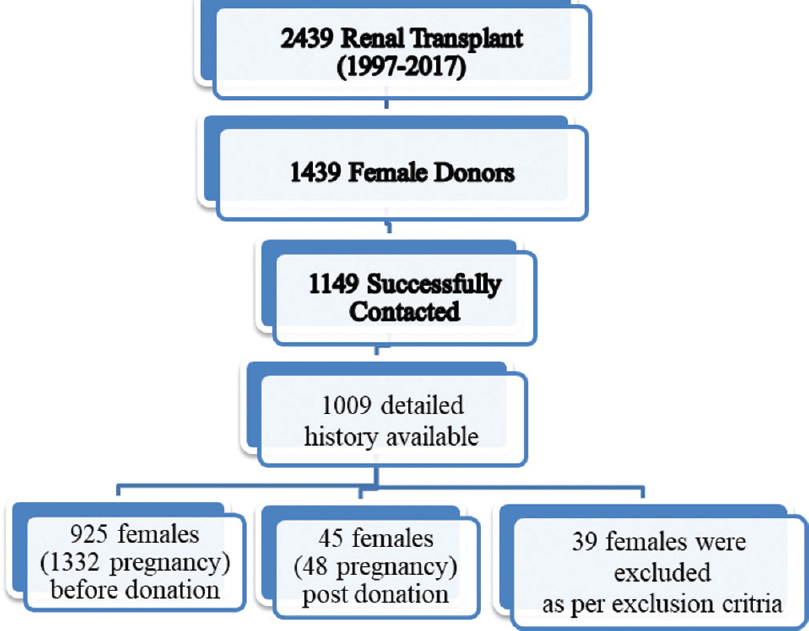
- Conceptual framework of the selection of study participants
Data collection- The baseline data and characteristics were obtained from all females who had consented to the study. Time (years) from 1st pregnancy to survey was 13.2 ± 2.6 years among the pre-donation group, while it was 5.2 ± 1.2 years in the post-donation group. Among the donor group, the baseline parameters—age; body weight, donation type (such as related or unrelated), donor ABO blood type, pregnancy details, and maternal and fetal outcomes—were noted from the available records.
The clinical obstetric outcomes among the kidney donors were collected, including the number of pregnancies, year of pregnancy, age at donation and delivery, height, and body weight at time of delivery. To evaluate maternal outcomes, the presence of proteinuria, gestational hypertension, preeclampsia events, and cesarean section was noted. Proteinuria was defined by positive results from a urine dipstick. A quantification of >300 mg/24 h was considered significant.
An obstetrician diagnosed preeclampsia at the time of delivery. Preeclampsia, typically defined as hypertension associated with new-onset proteinuria and edema, preterm birth, fetal growth restriction, and fetal death were the primary fetal outcomes. Gestational age of less than 37 weeks was defined as a preterm birth; fetal body weight of less than 2.7 kg was defined as indicative of fetal growth restriction.
Among the control group, details about maternal and fetal outcomes from medical records were obtained. Details about the time of delivery—age, body mass index (before and after pregnancy), medication history, history of gestational hypertension, history of overt or gestational diabetes, multipara, multigravida, delivery method, and pregnancy and fetal outcomes—were noted.
Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as frequency (%). We used independent samples t test to compare the means, whereas % were compared using Chi-square test/Fisher exact test. Univariate and multivariate binary logistic regression analyses were used to calculate the odds ratio and adjusted odds ratio, respectively (with 95% confidence interval and P values). P < 0.05 was considered statistically significant. Statistical package for social sciences, version-23 (SPSS-23, IBM, Chicago, USA) was used for the statistical analysis.
Results
In the present study, 925 female kidney donors (1332 pregnancies) with pregnancy before donation have been included for the analysis. A total of 45 females (48 pregnancies) who had their pregnancies in the post-donation period were included as study group. The average age (years) at donation among the pre-donation and post-donation groups was 32.15 ± 2.34 and 23.12 ± 2.1, respectively. The mean age at first pregnancy was significantly lower (22.6 ± 1.6 vs. 25.94 ± 1.2, P < 0.001) in the pre-donation pregnancy as compared to the post-donation pregnancy group [Table 1]. The average age of last pregnancy among the pre-donation group was 24.5 ± 1.7 years, with 56% having two issues, while 3% had more than two issues.
| Characteristics | Pre-donation Pregnancy (n=1332) | Post-donation Pregnancy (n=48) | P |
|---|---|---|---|
| Number of donors | 925 | 45 | <0.001 |
| Age (Years) at Pregnancy | 22.6±1.6 | 25.94±1.2 | <0.001 |
| eGFR (mL/min/1.73 m2) at donation | 77.2±11.6 | 78.1±10.8 | 0.597 |
| eGFR (mL/min/1.73 m2) at time of pregnancy | 65.2±10.3 | 66.5±9.8 | 0.587 |
| Serum Creatinine at time of pregnancy | 0.84±0.12 | 0.85±0.13 | 0.571 |
| Related donation | 574 (62.1%) | 13 (20.8%) | <0.001 |
| Weight (kg) gain during pregnancy | 8.12±1.2 | 8.82±1.2 | 0.001 |
| Prenatal checkup | 945 (70.9%) | 48 (100%) | <0.001 |
| Pregnancies | |||
| 1 | 540 (41%) | 43 (90%) | <0.001 |
| 2 | 746 (56%) | 4 (8%) | |
| 3 | 36 (3%) | 1 (2%) |
Data Presented in Mean±Standard Deviation/Frequency (%) compared by Independent samples t test/Chi-square test. P <0.05 significant
There was a significantly lower weight (kg) gain (8.12 ± 1.2 vs. 8.82 ± 1.2, P = 0.001), proportion of history of prenatal check-up (70.9% vs. 100%, P < 0.001), history of institutional delivery (26.7% vs. 91.6%, P < 0.001) and history of related donors (62.1% vs. 20.8%, P < 0.001) among the pre-donation pregnancy group as compared to the post-donation pregnancy females. Of the 48 pregnancies in the post-donation group, 4 had in-home delivery, 24 pregnancies underwent vaginal delivery, and cesarean delivery was conducted in 20 pregnancies. However, of 355 pregnancies that had institutional delivery in the pre-donation group, 3.8% underwent cesarean section. The remaining variables (body mass index, serum creatinine, and eGFR) were same between the two groups. There were higher proportions of ≥2 pregnancies (59% vs. 10%, P < 0.05) in the pre-donation group as compared to post-donation females [Table 1].
On decade-wise evaluation, of the 48 post-donation pregnancies, 20 occurred in 1997–2007 whereas 28 occurred in 2007–2017. In these patients, prematurity (90% vs. 42.85%, P = 0.001) and fetal loss (5% vs. 0%, P = 0.937) was insignificantly higher in the first decade (1997–2007) as compared to second decade (2007–2017).
The outcome of the 1332 before donation pregnancies was compared with post donation pregnancies. Results showed that the proportion of pre-eclampsia, gestational hypertension, gestational diabetes, post-partum hemorrhage, and full-term baby was insignificantly higher, whereas preterm birth with gestation of <37 weeks and fetal death was significantly lower in the post-donation pregnancy as compared to the pre-donation kidney women. Cesarean delivery (3.8% vs. 39.6%, P < 0.05), proteinuria (0.08% vs. 2.1%, P < 0.05), low birth weight (2.6% vs. 20.8%, P < 0.05) were significantly lower in the pre-donation pregnancy as compared to post-donation pregnancies [Table 2].
| Outcomes | Pre donation Pregnancy (n=1332) | Post donation Pregnancy (n=48) | P | OR (95% CI) |
|---|---|---|---|---|
| Pre-eclampsia | 1 (0.007%) | 0 | NS | 0.9 (0.3-2.5) |
| Gestational hypertension | 1 | 0 | NS | 0.9 (0.3-2.5) |
| Gestational Diabetes | 1 | 0 | NS | 1.2 (0.2-2.7) |
| Cesarean section | 45 (3.8%) | 19 (39.6%) | 0.001 | 17.9 (9.3-34.3) |
| Post-partum hemorrhage | 5 (0.4%) | 0 | 0.671 | -- |
| Proteinuria | 1 (0.08%) | 1 (2.1%) | <0.001 | 28.3 (1.8-459.7) |
| Full term | 1270 (95.3%) | 44 (91.6%) | 0.241 | 0.53 (0.2-1.5) |
| Low birth weight <2500 g | 34 (2.6%) | 10 (20.8%) | <0.001 | 10.1 (4.6-21.8) |
| Preterm birth with gestation of <37 weeks | 60 (4.2%) | 3 (6.2%) | 0.569 | 1.4 (0.4-4.7) |
| Fetal death | 27 (2.0%) | 1 (2.1%) | 0.960 | 1.03 (0.14-7.72) |
Chi square test/Fisher exact test used. Univariate binary logistic regression analysis used to compute odds ratio. P<0.05 significant
Binary logistic regression analysis was used to identify the determinants of the women who had post-donation pregnancies. In univariate analysis, cesarean delivery, proteinuria, and low birth weight (<2500 g) were significantly associated with post-donation pregnancy. In multivariate analysis, cesarean delivery (adjusted odds ratio: 8.2, 95% CI: 4.7–14.3, P = 0.024), and low birth weight (<2500 g) (adjusted odds ratio: 6.3, 95% CI: 2.3–11.8, P = 0.003) were significant higher in post donation pregnancy [Figure 2].
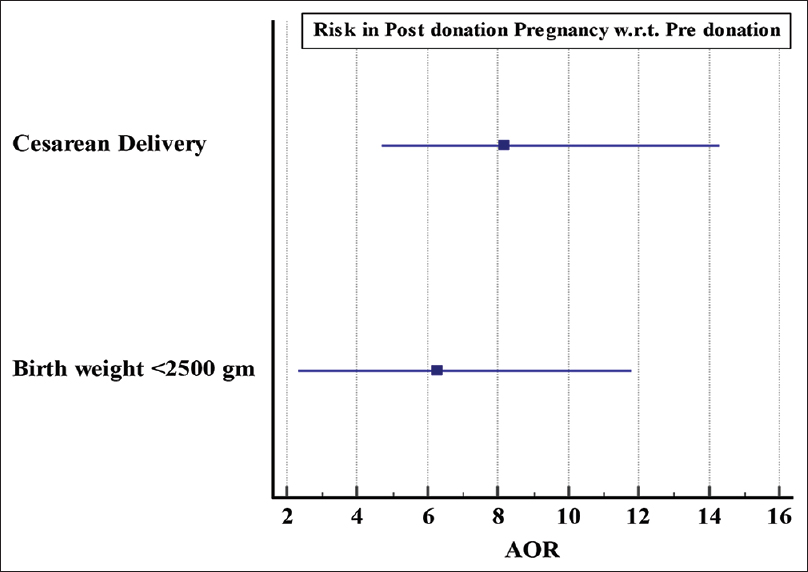
- Forest plot showing the independent risk factors of pregnancy outcomes in post-donation pregnancy w.r.t pre-donation pregnancy
In univariate analysis, age (years) at pregnancy, history of pre-natal checkups, time (years) since last pregnancy, and cesarean delivery were significantly associated with post-donation pregnancy, whereas low birth weight (<2500 g) was marginally associated (P = 0.058). In multivariate analysis, only cesarian delivery was significant higher (adjusted odds ratio: 2.1, 95% CI: 1.1–6.8, P = 0.024) whereas low birth weight (<2500 g) was insignificantly high (adjusted odds ratio: 3.3, 95% CI: 0.85–9.6, P > 0.05) in post-donation pregnancies[Table 3].
| Characteristics | Adjusted Odds ratio | P | ||
|---|---|---|---|---|
| Value | 95% CI | |||
| Lower | Upper | |||
| Post donation Pregnancy (vs. Pre-donation) [1332/48] | ||||
| Cesarean Delivery | 8.2 | 4.7 | 14.3 | 0.024 |
| Low birth weight <2500 g | 6.3 | 2.3 | 11.8 | 0.003 |
Multivariate binary logistic regression analysis used, P<0.05 significant
Discussion
The study demonstrated that the post-donation pregnancy is associated with low risk to maternal outcomes; however, there was an increased risk to fetal outcomes in terms of prematurity and low-birth-weight babies despite better antenatal checkups.
Earlier studies by Buszta et al.[6] Jones et al.,[7] and Wrenshall et al.[8] also did not show an increase pregnancy risk or subsequent renal impairment following pregnancy among kidney donors. There was no evidence of maternal complications of persistent hypertension, proteinuria, or deterioration in renal function or fetal abnormalities. However, recent studies by Ibrahim et al.[9] and Reisaeter et al.[10] reported a higher risk of maternal and fetal adverse outcomes among post donation pregnancies with gestational diabetes (2.7 vs. 0.7%), gestational hypertension (5.7 vs. 0.6%), proteinuria (4.3 vs. 1.1%), preeclampsia (5.5 vs. 0.8%), prematurity (7.1 vs. 4%), and fetal loss (19.2 vs. 11.3%). O'Keeffe et al.[12] calculated risk pooled estimates of pregnancy-related outcomes from the Ontario and Norwegian studies and found that pre-eclampsia was twice as frequent in donors as in controls (5.9 vs. 3.1%) with a relative risk of 2.12 (1.06–4.27). In our study population, we found an insignificant rise of preeclampsia, gestational hypertension, or diabetes in the post-donation group, though we observed a significant higher risk to prematurity and low-birth-weight babies. Following donor nephrectomy, our study population demonstrated a higher occurrence of proteinuria during pregnancy (2.08%) as compared to the pre-donation group (0.007%). This could be due to glomerular hyperfiltration as evidenced in literature favoring[313] the severe deleterious long-term effects of consecutive pregnancies on the glomeruli without underlying renal compromise.[4] Another probable explanation could be the one kidney model leading to hyper filtration resulting at times in mild proteinuria. The additional stress of pregnancy could add on to this hyperfiltration theory and thus possibly worsening the proteinuria. Previous studies have suggested preeclampsia as a risk to post-donation pregnancy in view of proteinuria. This proteinuria could have been misinterpreted as pre-eclampsia as none of the above studies could confirm these findings with biomarkers such as fms-like tyrosine kinase 1 (sFlt-1) or kidney biopsy, thus suggesting just proteinuria and not exactly preeclampsia.[1415] Fisher et al.'s study[16] showed that only 55% of women suspected of having preeclampsia had evidence of preeclampsia on kidney biopsy underscoring the difficulty of accurate diagnosis. In our study population, post-donation pregnancies females were older (25.94 ± 1.2) years to the pre-donation group (22.6 ± 1.6 years). However, these women were not as old as to be at a major risk to pre-eclampsia. Garg et al.[11] in their subgroup analysis showed that women with age >32 years were at increased risk to gestational hypertension and/or pre-eclampsia; definitely, our post-donation females were much younger at time of conception. The conventional risk factors to preeclampsia and or gestational hypertension are hypertension, diabetes, higher BMI, being nulliparous, history of preeclampsia in a previous pregnancy, family history of preeclampsia, multiple pregnancies, and maternal age more than 40 years.[1718]. However, the post-donation females observed lower BMI (21.45 ± 1.23), no significant weight gain during pregnancies, no previous history or family history of preeclampsia or history of multiple pregnancies (10.4%), and the results were comparable between the two groups. Thus, despite the other major studies showing higher BMI (23.6) and multiparity in nearly 49.2% of the study group, our study group did not substantiate their findings. Most of the post-pregnancy donors (35; 72.9%) in our study group were unrelated while among related studies,[91011] majority (82%–96.2%) of them were related donors. There could be a possibility that some familial association for hypertension among these cohort population related to the recipient suggesting -genetic predisposition to underlying renal disease, which then could have become more pronounced after donation and pregnancy, acting as a second hit leading to hyperfiltering kidney with proteinuria. Another risk factor to preeclampsia is the higher age at time of conception and longer duration between two pregnancies; both were much lower in our study group.
The previous studies of post-donation pregnancy[91011] outcomes have mainly involved the Caucasian populations who are economically well placed with higher age of conception,[9101119] Pregnancy complications even among the general population are more common in African Americans and Caucasians than among Asians. Since overall incidence of preeclampsia and gestational hypertension is very low among Asian community especially among Indians,[20] and age of conception are often lower, thus generalization of above results among Asian populations needs to be seen with caution.[20] Thus, Indian data needs to be generated to resolve this issue among our donors who are younger and less predisposed as compared to western population.
Our study population showed a higher occurrence of prematurity and low birth weight among the post-donation group, which was substantiated by Garg et al. and others in the literature.[91121] Despite good antenatal checkups of post donation pregnancies (50% vs. 28%), higher fetal complications were observed. Undernourished low BMI mothers, poor weight gain during pregnancies, low socioeconomic population despite more vigilant and close watch during pregnancy (history of kidney donation and fear among the treating doctors of donor pregnancies), and fetal complications were higher. Besides, the countries' limited healthcare access, could be possible reasons for higher fetal complications, including prematurity, low birth weight, and increased number of cesarean section. We need more data to confirm these observations and to better understand who all are at risk.
The limitation of our study is the smaller post-donation population as the majority (91.6%) of our population had pre-donation pregnancies. The data was based on the questionnaire filled in by the donor responses, which was not verified against hospital or clinic records; therefore, this data must be interpreted with great caution. A large population in our study group did not respond to the questionnaire, and among those who responded, 12.2% had no details about their pregnancy status, thus undermining our results.
Conclusions
Our data shows that post-donation pregnancies appear to have minimal risk to maternal outcomes but a slightly higher risk for low birth weight and prematurity as compared to the pre-donation pregnancies. It seems a large prospective national registry should be instituted to follow all kidney donors.
Declaration- Disclosure
The article has been approved by the institutional ethical committee. All authors have given consent for publication.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303:959-66.
- [Google Scholar]
- Morbidity and mortality in 1022 consecutive living donor nephrectomies: Benefits of a living donor registry. Transplantation. 2009;88:1273-9.
- [Google Scholar]
- Impact of the donor-recipient gender matching on the graft survival from live donors. BMC Nephrol. 2020;21:5.
- [Google Scholar]
- Physiological and biochemical effects of pregnancy in uninephrectomized rabbits. Hypertens Pregnancy. 1991;10:35-48.
- [Google Scholar]
- Pregnancy and birth after kidney donation: The Norwegian experience. Am J Transplant. 2009;9:820-4.
- [Google Scholar]
- Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372:124-33.
- [Google Scholar]
- Mid- and long-term health risks in living kidney donors: A systematic review and meta-analysis. Ann Intern Med. 2018;168:276-84.
- [Google Scholar]
- Effects of a reduction in maternal renal mass on pregnancy and cardiovascular and renal function of the pregnant women. Am J Physiol Ren Physiol. 2006;290:F1153-62.
- [Google Scholar]
- Predictive value of the sFlt-1: PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13-22.
- [Google Scholar]
- Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992-1005.
- [Google Scholar]
- Hypertension in pregnancy: Clinical-pathological correlations and remote prognosis. Medicine (Baltimore). 1981;60:267-76.
- [Google Scholar]
- Risk factors for pre-eclampsia at antenatal booking: Systematic review of controlled studies. BMJ. 2005;330:565.
- [Google Scholar]
- Racial/ethnic disparities in obstetric outcomes and care: Prevalence and determinants. Am J Obstet Gynecol. 2010;202:335-43.
- [Google Scholar]
- Preeclampsia: Disease biology and burden, its management strategies with reference to India. Pregnancy Hypertens. 2019;15:23-31.
- [Google Scholar]







