Translate this page into:
Acute Kidney Injury Complicating Severe Acute Pancreatitis: Clinical Profile and Factors Predicting Mortality
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Acute kidney injury (AKI) frequently complicates severe acute pancreatitis (SAP) among the critically ill. We studied clinical profile and risk factors predicting mortality in SAP-AKI.
Materials and Methods:
We conducted a prospective observational study of 68 patients with SAP-AKI from September 2015 to September 2019. Patient data and outcomes grouped as survivors and deceased were analyzed.
Results:
SAP-AKI constituted 2.14% (68 of 3,169) of all AKIs with 1.5%, 20.6%, and 77.9% in Kidney Disease Improving Global Outcomes (KDIGO) Stages I, II and III respectively. The mean age was 39.93 ± 11.79 years with males 65 (95.6%). The causes of acute pancreatitis were alcohol addiction 59 (86.8%), highly active antiretroviral therapy 1 (1.4%), hypercalcemia 1 (1.4%), IgG4-related disease 1 (1.4%), and unidentified 6 (8.8%). Complications were volume overload, shock, respiratory failure, and necrotizing pancreatitis in 21 (30.9%), 10 (14.7%), 6 (8.8%), and 14 (20.5%), respectively. Kidney replacement therapy done in 40 (58%), with intermittent hemodialysis 36 (53%) and acute intermittent peritoneal dialysis 4 (6%). The overall mortality was 23 (33.8%), three progressed to chronic kidney disease (thrombotic microangiopathy 2; biopsy inconclusive 1). In 45 survivors, AKI recovered in 22.7 ± 9.6 days. Death occurred within first 6 days. The risk factors associated with in-hospital mortality was necrotizing pancreatitis (odds ratio [OR] = 5.143; 95% confidence interval 1.472–17.972; P = 0.01), circulatory failure (OR = 6.125; P = 0.016), peak creatinine >3 mg/dL (OR = 7.118; P = 0.068), Bedside Index of Severity for Acute Pancreatitis score >3 (OR = 8.472; P = 0.001), need for kidney replacement therapy (OR = 3.764; P = 0.024), KDIGO III (OR = 9.935; P = 0.03).
Conclusions:
Alcohol addiction was the commonest cause of severe acute pancreatitis. The overall mortality was 33.8%. Necrotizing pancreatitis, circulatory failure, peak creatinine >3 mg/dL, BISAPs >3, KDIGO III, and the need for kidney replacement therapy were independent risk factors for mortality.
Keywords
Acute kidney injury
BISAP score
kidney replacement therapy
severe acute pancreatitis
thrombotic microangiopathy
Introduction
Acute pancreatitis (AP) is an inflammatory condition involving the pancreas with severity ranging from mild disease to severe acute pancreatitis (SAP). SAP is an acute clinical syndrome with multiple organ failure and high mortality in the range of 7% to 47%.[1] Acute kidney injury (AKI) is a frequent complication of SAP carrying a dismal prognosis, when kidney replacement therapy is required, with mortality ranging from 25% to 75%.[2] A ten-fold increase in mortality has been shown to be associated with AKI in pancreatitis.[3] Retrospective data by Kumar et al.[1] and Manokaran et al.[4] reported SAP-AKI in 19.4% and 32% with a mortality of 57.1% and 12.5%, respectively. However, SAP-AKI is greatly understudied, as the clinical characteristics and underlying processes of AKI coexisting with SAP stay elusive. Hence, we conducted a prospective study to determine the complications, factors predicting mortality, and renal outcomes among SAP-AKI.
Materials and Methods
We prospectively analyzed (n = 68) patients with SAP-AKI admitted at Madras Medical College between September 2015 and September 2019 in our tertiary care center. The inclusion criteria were as follows: patients with AKI as per Kidney Disease: Improving Global Outcomes (KDIGO)[5] staging presenting with clinical, biochemical, and imaging criteria for acute pancreatitis (ICD-9 [International Classification of Diseases, Ninth Revision]; Code 577). The exclusion criteria were as follows: patients with chronic pancreatitis and known preexisting kidney disease. Demographic, clinical, laboratory, and imaging data were obtained. AP was diagnosed in patients who met two of the following three criteria: (1) abdominal pain (acute onset of a persistent, severe, epigastric pain often radiating to the back), (2) serum lipase/amylase levels at least three times greater than the upper limit of normal, and (3) imaging evidence of AP. SAP was defined as per Revised Atlanta classification criteria-2012 of persistent organ failure >48 hours and local complications (peripancreatic fluid collection, pseudocyst, necrotic collection, and walled-off necrosis).[6] Bedside Index of Severity for Acute Pancreatitis score (BISAPs)[7] defined by blood urea nitrogen >25 mg/dL, impaired mental status, systemic inflammatory response syndrome (SIRS) ≥2/4 present, age >60 years, and pleural effusion – a scoring tool to assess the probability of in-hospital mortality within 24 hours of presentation was applied. Kidney replacement therapy (KRT) was done when indicated. Renal biopsy was done in cases with AKI persisting more than 2 weeks. The cohorts were grouped as survivors and deceased [Figure 1]. The survivors were followed every 3 months, and blood pressure, urine analysis, urine spot protein–creatinine ratio (PCR), and serum creatinine tests were done during the visits. Glomerular filtration rate was estimated using Chronic Kidney Disease – Epidemiologic Collaboration Creatinine (CKD-EPI) equation (2009). The study protocol was approved by the Institutional Ethics Committee, Madras Medical College, registration number- ECR/270/inst./TN/2013/RR-16.

- Flow of the Study
Statistical analysis
The continuous variables were described as mean with standard deviation/median and interquartile range (IQR) as per normality of data. The categorical variables were described as frequencies and percentages. Comparisons between continuous groups were done by Student's t tests or Mann–Whitney U tests and categorical groups by Chi-square or Fisher's exact tests according to the distribution. Multinomial logistic regression was applied to predict the independent risk factors for mortality. Risk factors that were found to be significant in univariate analysis were considered in multiple regression models. A two-sided P value < 0.05 was considered significant. The analysis was done by SPSS Version 23.0 (SPSS Inc., IBM, USA).
Results
A total of 68 patients with diagnosis of SAP-AKI were included during the study period. This constituted 2.14% (68 of 3,169) of all-cause AKIs. Basic demographic details are shown in Table 1. Oliguria (urine output <500 mL/day) was noted in n = 40 (58.8%). Volume overload in 21 (30.8%; P = 0.031), shock in 10 (14.7%; P = 0.014), and respiratory failure requiring mechanical ventilation in 6 (8.8%; P = 0.001) were associated with significant mortality. Imaging noted in SAP were edematous interstitial pancreatitis in n = 53 (77.9%), necrotizing pancreatitis in 14 (20.6%), emphysematous pancreatitis in 1 (1.4%), and pseudocyst in 7 (10.3%). Necrotizing pancreatitis (39% vs. 11%, P = 0.01) was associated with significant mortality. BISAPs >3 (86.3% vs. 60%, P = 0.038) predicted increased mortality. An ROC (receiver operating characteristics) on BISAPs was performed, with sensitivity of 97.8% and specificity of 52.2% (area under the curve [AUC] = 0.728 [0.587–0.869]; P = 0.002) for predicting mortality in SAP-AKI in our cohort [Figure 2]. The mean time to AKI occurrence from symptom onset is 2.58 ± 3.4 days. KDIGO Stage I in n = 1 (1.5%), KDIGO Stage II in 14 (20.6%), and KDIGO Stage III in 53 (77.9%), with higher KDIGO stage (95.6% vs. 68.8%, odds ratio [OR] = 9.935; 95% confidence interval [CI; 1.215–81.219]; P = 0.03) predicting mortality. Seven patients had clinical evidence of thrombotic microangiopathy (TMA) in the form of hemolytic anemia, raised lactate dehydrogenase, peripheral smear suggesting schistiocytes (>5%), and new onset or worsening hypertension, of which three had biopsy-proven TMA. KRT was done in 40 (59%), among which hemodialysis in 36 (90%) and 4 (10%) required acute intermittent PD, an increased need for KRT (78.2% vs. 48.8%; OR = 3.764; 95% CI [1.191–11.891]; P = 0.024) was seen among the deceased population. The local and systemic complications are given in Table 2. The occurrence of hyperamylasemia (> 350 IU/mL; P = 0.02), serum calcium <8 mg/dL (P = 0.03), hypoalbuminemia (P = 0.05), international normalized ratio (INR) > 1.1 (P = 0.014) at presentation predicted risk of mortality. By logistic regression, the occurrence of necrotizing pancreatitis, circulatory failure, peak creatinine >3 mg/dL, BISAPs >3, need of KRT, and KDIGO stage III independently predicted the risk for mortality [Table 3]. We reported 23 (33.8%) deaths during hospital stay. The deceased patients had a shorter median intensive care unit length of stay (LOS; 5 vs. 10; P < 0.001).
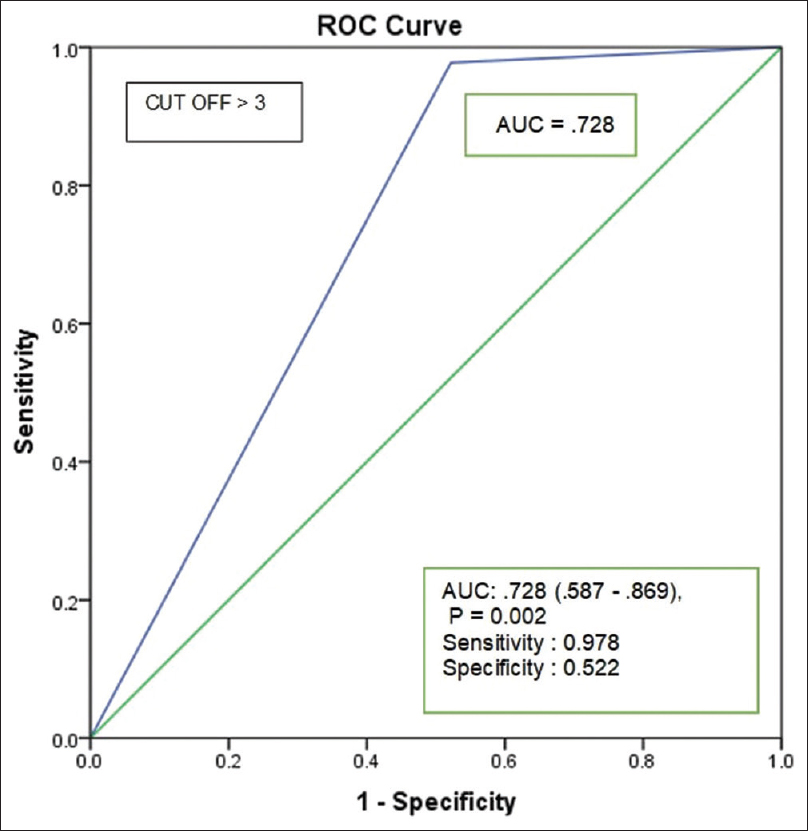
- BISAPs (receiver operating characteristics) for predicting mortality. BISAPs = Bedside Index of Severity for Acute Pancreatitis score
| Characteristics | Total (n=68) | Survivors (n=45, 66.2%) | Deceased (n=23, 33.8%) | P |
|---|---|---|---|---|
| Mean age (SD), yearsa | 39.93±11.78 | 39.8±11.48 | 40.17±12.61 | 0.06 |
| Sex, n (%) | ||||
| Male | 65 (95.6%) | 42 (93.5%) | 23 (100%) | - |
| Female | 3 (4.4%) | 3 (6.7%) | 0 | |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 5 (7.4%) | 4 (8.9%) | 1 (4.3%) | |
| Hypertension | 7 (10.3%) | 6 (13.3%) | 1 (4.3%) | -- |
| Alcoholism | 59 (86.8%) | 37 (82.3%) | 22 (95.6%) | |
| Cholelithiasis | 3 (4.6%) | 2 (4.4%) | 1 (4.3%) | |
| Hypertriglyceridemia | 4 (5.8%) | 2 (4.3%) | 2 (8.6%) | |
| Etiology, n (%) | ||||
| Alcoholism | 59 (86.8%) | |||
| HAARTf | 1 (1.4%) | |||
| IgG4 disease | 1 (1.4%) | |||
| Hypercalcemia | 1 (1.4%) | |||
| Unidentified | 6 (8.8%) | |||
| BISAPs >3 | 46 (67.6%) | 27 (60%) | 19 (86.3%) | 0.038 |
| Time to AKI (days)a | 2.58±3.40 | 3.09±4.06 | 1.61±0.98 | 0.98 |
| KDIGO Staged | ||||
| Stage I | 1 (1.5%) | 1 (2.2%) | 0 | 0.03 |
| Stage II | 14 (20.6%) | 13 (28.8%) | 1 (4.4%) | |
| Stage III | 53 (77.9%) | 31 (68.8%) | 22 (95.6%) | |
| KRTe | ||||
| Yes | 40 (58.8%) | 22 (49%) | 18 (78.2%) | 0.02 |
| No | 28 (41%) | 23 (51%) | 5 (22%) | |
| Hemoglobin (g/dL)b | 11.53±2.78 | 11.26±3.02 | 12.04±2.21 | 0.28 |
| Urea (mg/dl)a | 125.22±56.83 | 120.93±62.49 | 133.43±44.15 | 0.30 |
| Peak creatinine >3 mg/dL | 56 (82.3%) | 34 (75.5%) | 22 (95.6%) | 0.04 |
| Serum LDH (IU/mL)a | 450±1866 | 1150±2196 | 538.06±463.92 | 0.27 |
| Serum amylase (IU/mL)b | 578 (228-1,060) | 451.4 (152.25-981.25) | 891 (454-1,267) | 0.02 |
| Serum lipase (IU/mL)a | 857±800.6 | 705.86±654.54 | 1133.22±971.07 | 0.06 |
| Serum calcium <8 mg/dL | 26 (38.2%) | 14 (31%) | 12 (52%) | 0.04 |
| ALT (IU/mL)b | 40 (20-67) | 24 (16-55) | 50 (30-119) | 0.005 |
| AST (IU/mL)b | 30 (17-56) | 27 (15.5–44.75) | 48 (17-80) | 0.02 |
| Albumin (g/dL)a | 2.94±0.62 | 3.05±0.68 | 2.73±0.39 | 0.05 |
| INRc | 1.1 (1-1.8) | 1.1 (1-1.8) | 1.3 (1-1.7) | 0.004 |
aMean, standard deviation (SD); bMedian, interquartile range (IQR); cMode (minimum–maximum); dKidney Disease Improving Global Outcomes; eKidney replacement therapy; fHighly active antiretroviral therapy. BISAPs=Bedside Index of Severity for Acute Pancreatitis score; AKI=acute kidney injury; LDH=lactate dehydrogenase; ALT=alanine transaminase; AST=aspartate transaminase; INR=international normalized ratio
| Characteristics | Total (n=68) | Survivors (n=45) | Deceased (n=23) | P |
|---|---|---|---|---|
| Necrotizing pancreatitis | 14 (20.5) | 5 (11) | 9 (39) | 0.01 |
| Interstitial edematous pancreatitis | 53 (77.9) | 39 (86.6) | 14 (60.8) | 0.21 |
| Emphysematous pancreatitis | 1 (1.4) | 1 (2.2) | 0 | — |
| Pseudocyst | 7 (10.2) | 5 (11.1) | 2 (8.6) | 0.31 |
| Volume overload | 21 (30.9) | 10 (22.2) | 11 (47.8) | 0.03 |
| Circulatory failure | 10 (14.7) | 3 (6.7) | 7 (30.4) | 0.01 |
| Respiratory failure | 6 (8.8) | 0 | 6 (26.1) | 0.001 |
| Variables | ORa | 95% CIb | P |
|---|---|---|---|
| Necrotizing pancreatitis | 5.143 | 1.472-17.972 | 0.01 |
| Circulatory failure | 6.125 | 1.408-23.628 | 0.01 |
| BISAPs > 3 | 8.472 | 2.359-30.480 | 0.008 |
| Peak creatinine >3 mg/dL | 7.118 | 0.858-59.073 | 0.06 |
| KRT | 3.764 | 1.191-11.891 | 0.02 |
| AKI Stage III | 9.935 | 1.215-81.219 | 0.03 |
aOdds ratio; b95% confidence interval. BISAPs=Bedside Index of Severity for Acute Pancreatitis score; KRT=kidney replacement therapy; AKI=acute kidney injury
Among the total survivors (n = 45) at discharge, 16 followed up as per protocol with median duration of 3.5 months. The mean time to nadir creatinine was 22.7 ± 9.6 days. Of these, three progressed to chronic kidney disease (CKD), one in CKD Stage 2 and the other two in CKD Stage 4.
Renal biopsy was done in seven patients [Table 4], three had TMA [Pictures 1 and 2], of which one developed thrombotic thrombocytopenic purpura (TTP) with bilateral cortical blindness due to bilateral lateral geniculate bodies (LGB) infarction, requiring plasma exchange with complete recovery. ADAMTS 13 activity was normal. Two had nonrecovery progressing to CKD Stage 2 and Stage 4 and the other in CKD Stage 4 with inconclusive pathology.
| Age | sex | Comorbid | Etiology | Peak Sr. Creatinine (mg/dL) | Biopsy | KRT (HD/PD) | Hospitalization; LOS | eGFR(ml/min/1.73m2)(follow-up) | Others |
|---|---|---|---|---|---|---|---|---|---|
| 35 | M | Prior pancreatitis | Alcoholism | 12.0 | TMA (picture 1) (L C3, N C4) | Yes | 15 | 51 | — |
| 43 | M | Hypertension | Alcoholism | 8.7 | TMA (picture 2) (N C3, N C4) | Yes | 29 | 28 | — |
| 26 | M | Nil | Idiopathic | 6.1 | TMA (ADAMTS13 – .76{0.4-1.2 IU/Ml}) | 1 HD/1 PLEX | 25 | 101 | Cortical blindness (B/L LGB infarction) |
| 45 | M | Diabetes mellitus | Alcoholism | 6.0 | ATI/Pigment Casts | Yes | 15 | 72 | — |
| 46 | M | Nil | Alcoholism | 6.4 | ATI/Rhabdomyolysis | Yes | 18 | 90 | — |
| 44 | M | Hypertension | Alcoholism | 11.0 | Biopsy inconclusive (IgA deposition) | Yes | 10 | 26 | — |
| 33 | M | Hypertension | Nil | 9.1 | IgG4 disease | Yes | 10 | Lost FU | — |
eGFR=estimated glomerular filtration rate; M=Male; F=female; TMA=thrombotic microangiopathy; IFTA=interstitial fibrosis and tubular atrophy; TTP=thrombotic thrombocytopenic purpura; ATI=acute tubular injury; HD=hemodialysis; PD=peritoneal dialysis; PLEX=plasma exchange; LOS=length of stay, LGB=lateral geniculate bodies; FU=follow-up, C3=Complement 3, C4=Complement 4, N=normal, L=Low; Ig=immunoglobulin
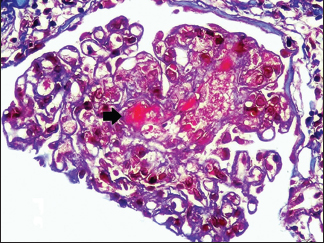
- Fibrin thrombi seen occluding the capillary lumen (MT 400x,
RENOPATH, Chennai)
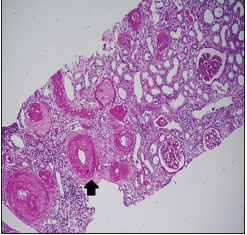
- Fibrin thrombi seen occluding the arterioles, with fibrous intimal
proliferation (PAS 100x. RENOPATH, Chennai)
Discussion
AKI is a common complication in patients with SAP with incidence ranging from 14% to 42%.[38] In our study, age and gender did not correlate with mortality. Li et al.,[8] Zhou et al.,[9] and Devani et al.[10] reported a positive correlation with age and female gender with mortality. The occurrence of comorbidities in our study was not associated with mortality, which was similar to study done by Zhou et al.[9]; however, Kumar et al.[1] and Manokaran et al.[4] reported alcohol addiction and diabetes mellitus as significant risk factors for death. We reported chronic and binge alcohol intake as the commonest etiology, which was similar to Kumar et al.[1]; however, Company et al.[11] and Li et al.[8] reported biliary disease, followed by alcohol. Gougol et al.[12] reported post-ERCP (endoscopic retrograde cholangiopancreatography) status, followed by alcohol addiction and biliary disease.
AKI is a major complication that occurs in patients with AP due to many factors such as hypovolemia due to vascular permeability and systemic inflammation, septic shock, vasoconstriction, abdominal compartment syndrome, and reduced tissue perfusion, which ultimately causes organ damage and multiorgan failure.[13] Several studies have asserted that renal failure is always one of the main drivers of multiorgan failure in SAP.[9] In our study, higher deaths occurred in AKI with volume overload, circulatory and respiratory failure. Gougol et al.[12] reported organ failure in 22% (111 of 500) presenting with AP, among which 15% (17 of 111) had isolated renal failure. Kumar et al.[1] and Zhou et al.[9] in their study reported shock (9 vs. 0; P < 0.001;OR = 202) and acute respiratory distress syndrome (P = 0.009) with mortality, respectively.
The BISAPs has been developed to identify patients at risk for mortality or severe disease early during the course of AP. In our study, a BISAPs >3 was significantly associated with mortality with a sensitivity of 97% and specificity of 52%, predicting the probability of death. In a meta-analysis by Gao et al.[7] stated that BISAPs >3 had an overall sensitivity of 56%, with a specificity 91% for mortality. Our results established higher amylase >350 IU/mL, severe hypocalcemia (<7 g/dL), hypoalbuminemia (serum albumin <3.5 mg/dL), peak creatinine >3 mg/dL, deranged coagulation at admission with mortality. Manokaran et al.,[4] in their study of 32 patients with SAP-AKI, reported that serum amylase >200 IU/mL and serum creatinine >2.4 mg/dL had four times increased risk of mortality compared to without AKI.
In our study, KDIGO Stage III had the highest mortality similar to Zhou et al.[9] Our results showed KRT in 59%, higher in deceased 78.2%, similar to Zhou et al.[9] with 414 patients wherein KRT was done in 59% (245), with 69% (89) among the deceased. Manokaran et al.[4] reported that 6.25% (2 of 32) required KRT. We reported an overall mortality of 23 (33.8%), similar to Zhou et al.,[9] Company et al.,[11] Kong et al.[14] and Kumar et al.[1] reported mortality of 44.9%, 30.5%, 31%, and 57.1%, respectively, in SAP-AKI. The major cause of death may be due to sepsis and multiorgan failure. TMA was the common finding in our cohort, with seven patients having clinical TMA, of which three underwent kidney biopsy. All of them developed TMA after SAP, pointing to secondary HUS rather than aHUS with pancreatic involvement. In a systematic review, Chaudhry and Sawier[15] showed 27 cases with TMA preceded by AP described in the literature, the mechanism of which is less clearly understood.
Thachil[16] explained that inflammatory release of cytokines like interleukin 6 (IL-6), IL-1, IL-8, and tumor necrosis factor alpha (TNF-α) stimulates the release of ultra-large vWF (von Willebrand Factor) from endothelium leading to deficiency of ADAMTS-13 protease, which gets consumed quickly. Similarly, Swisher et al.[17] hypothesized the presence of circulating pancreatic protease-mediated direct endothelial injury modifying the circulating vWF to ultra-large vWF resistant to proteolytic cleavage or a relative deficiency of ADAMTS13 with subsequent platelet aggregation. Serum ADAMTS13 activity estimation was done in one of our patients with severe renal and neurological involvement who improved with plasma exchange despite having normal ADAMTS13 levels. Chaudhry and Sawier[15] even highlighted the ability of TTP to occur despite normal ADAMTS13 activity and suggested that a more complex model may underpin the pathologic process. Furthermore, the role of complement activation in SAP has been demonstrated in necrotizing pancreatitis models, wherein direct cleavage of C3 and C5 by pancreatic enzyme trypsin with significantly low C3 levels causes microvascular damage.[16] Similarly Ruiz-Torres et al.[18] established the role of complements and neutrophils in microvascular damage from sera from patients with TMA shown to cause C3 and membrane attack complex deposition and surface expression of P selectin on the human endothelial cell line. There is also evidence from animal models that pancreatic endothelial nitric oxide synthase is decreased in AP predisposing to the development of TMA.[19] Three patients progressed to CKD in the follow-up cohort.
There are certain limitations to our study. First, due to lack of exact and complete urine volumes, we relied on serum creatinine obtained during the relevant period and possibly could not include patients fulfilling AKI criteria with urine output. Second, risk factors for AKI in SAP could not be studied as patients having SAP without AKI were not included. Third, a relatively small sample size involving only single-center data cannot be extrapolated to the general population. Fourth, our long-term follow-up data remain insufficient as fewer patients reviewed stating logistic difficulties.
Conclusions
SAP with AKI constituted 2.14% of all-cause AKI. High mortality of 33.8% was seen. We found necrotizing pancreatitis, circulatory failure, peak creatinine >3 mg/dL, BISAPs >3, need for KRT, and KDIGO Stage III as significant risk factors associated with high mortality among SAP presenting with AKI. Three patients progressed to CKD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Acute kidney injury in severe acute pancreatitis: An experience from a tertiary care center. Saudi J Kidney Dis Transpl. 2015;26:56-60.
- [Google Scholar]
- Renal failure in acute pancreatitis. Timing of dialysis and surgery. Przegl Lek. 2000;57(Suppl 5):29-31.
- [Google Scholar]
- Acute renal failure complicating severe acute pancreatitis. Renal Fail. 1996;18:629-33.
- [Google Scholar]
- A study of acute kidney injury in severe acute pancreatitis in a tertiary care hospital from south India. IOSR-JDMS. 2018;17:45-8.
- [Google Scholar]
- Kidney disease improving global outcomes (KDIGO) 2012. Kidney International Supplements. 2012;2:8-12.
- [Google Scholar]
- Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-11.
- [Google Scholar]
- The value of BISAP Score for predicting mortality and severity in acute pancreatitis. A systematic review and meta-analysis. PLoS One. 2015;10:e0130412.
- [Google Scholar]
- Risk factors and outcome of acute renal failure in patients with severe acute pancreatitis. J Crit Care. 2010;25:225-9.
- [Google Scholar]
- Effect of AKI on mortality and hospital stay in patient with severe acute pancreatitis. Nephrology. 2015;20:485-91.
- [Google Scholar]
- Acute pancreatitis: Trends in outcomes and the role of acute kidney injury in mortality-A propensity-matched analysis Pancreatology. 2018;18:870-7.
- Factors predicting mortality in severe acute pancreatitis. Pancreatology. 2003;3:144-8.
- [Google Scholar]
- Clinical outcomes of isolated renal failure compared to other forms of organ failure in patients with severe acute pancreatitis. World J Gastroenterol. 2017;23:5431-7.
- [Google Scholar]
- Pathophysiology of acute kidney injury in severe acute pancreatitis-An overview. Gastroenterol Hepatol Open Access. 2019;10:242-5.
- [Google Scholar]
- Clinical characteristics and prognostic factors of severe acute pancreatitis. World J Gastroenterol. 2004;10:3336-8.
- [Google Scholar]
- Thrombotic thrombocytopenic purpura precipitated by acute pancreatitis. Transfusion Apheresis Sci. 2011;45:143-7.
- [Google Scholar]
- Lessons from acute pancreatitis – induced thrombotic thrombocytopenic purpura. Eur J Intern Med. 2009;20:739-43.
- [Google Scholar]
- Pancreatitis preceding acute episodes of thrombotic thrombocytopenis purpura – haemolytic uremic syndrome: Report of five patients with systematic review of published reports. Haematlogica. 2008;92:936-43.
- [Google Scholar]
- Complement activation: The missing link between ADAMTS13 deficiency and microvascular thrombosis of thrombotic microangiopathies. Thromb Haemost. 2005;93:443-52.
- [Google Scholar]
- Endothelial nitric oxide synthase in the initiation of caerulein- induced acute pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2004;287:G80-7.
- [Google Scholar]







