Translate this page into:
Intestinal Pseudo-Obstruction – An Under-Recognized Presentation of Systemic Lupus Erythematosus
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Intestinal pseudo-obstruction (IPO), characterized by obstruction without an identifiable anatomic cause, is a rare and poorly understood entity that may occur as a primary condition or secondary to other autoimmune disorders such as systemic lupus erythematosus (SLE). A12-year-old female child was brought with abdominal distension, vomiting, and fever for 15 days. Examination showed height and weight less than the third centile for age, tachypnea, tachycardia, and hypertension with severe abdominal distension, copious bilious aspirate, and very sluggish bowel sounds. Abdominal X-ray showed multiple air fluid levels. Ultrasound abdomen and unenhanced computed tomography (CT) scan revealed thickened dilated bowel loops, ascites, and pleural effusion. In view of multisystem nature of the disease, Koch's abdomen or autoimmune disease was suspected and emergency laparotomy procedure was deferred. She was evaluated and diagnosed to have SLE with lupus nephritis class V as per the International Society of Nephrology/Royal Pathology Society. She was managed conservatively with nasogastric decompression, immunosuppressive therapy and supportive hemodialysis.
Keywords
Hemodialysis
intestinal pseudo-obstruction
renal failure
systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disorder characterized by the production of autoantibodies directed against nuclear and cytoplasmic antigens.[1] The commonly involved organ systems include hematologic and musculoskeletal systems, kidneys, and the central nervous system. Gastrointestinal (GI) symptoms seen in 50% patients are usually mild or secondary effects to medication.[2] Intestinal pseudo-obstruction (IPO) has been reported rarely as the manifestation of pediatric SLE. Early recognition and prompt management of this complication are lifesaving and avoid unnecessary laparotomy.
Case Report
A 12-year-old female child was brought with complaints of fever, abdominal pain with distension, bilious vomiting, and constipation for 15 days and diminished urine output for 3 days. On examination, her height and weight were less than the third centile for age with pallor and pedal edema. Her heart rate was 104/minute, respiratory rate was 42/minute, and blood pressure was 132/90 mmHg (>95th percentile). Abdominal examination revealed distension with a dull percussion note and absent bowel sounds. Chest showed diminished breath sounds with occasional crackles on both sides. Cardiovascular and central nervous system examination was essentially normal. Radiograph showed multiple air fluid levels in the abdomen and mild bilateral pleural effusion [Figure 1]. Her investigations showed hemoglobin 7.3 g/dL, total leukocyte count 5600/mm3, platelet count 89,000/mm3, sodium 129 mEq/L, potassium 5.8 mEq/L, blood urea nitrogen 76 mg/dL, serum creatinine 3.2 mg/dL, serum albumin 2.3 g/L, erythrocyte sedimentation rate 115mm/h, and C-reactive protein negative. Urine routine and microscopy showed albumin 4+ with 50–70 red blood cells per high-power field (hpf), and 10–15 pus cells/hpf. Urine and blood culture were negative. Mantoux test was negative, and gastric lavage for acid-fast bacilli (AFB) was normal. Ultrasound abdomen and unenhanced computed tomography (CT) scan revealed moderate ascites, diffuse bowel wall thickening of duodenum and jejunum, and pleural effusion. Ascitic tap showed 78 cells/mm3 with 70% polymorphs, glucose 76 mg/dLand protein 1.8 g/dL, adenosine deaminase (ADA) 29 IU/L, and negative stain for AFB. In the setting of acute febrile illness with severe weight loss, renal and hematological involvement with GI symptoms with no laboratory evidence of sepsis, SLE was suspected. Surgery was deferred and conservative management of intestinal obstruction in the form of nil by mouth, nasogastric decompression, intravenous fluids, and antibiotics instituted. Further workup showed C3 10.1 mg/dL (normal 90–180 mg/dL), C4 3.2 mg/dL (normal 10–40 mg/dL), antinuclear antibody (ANA) 1:1000, and anti-double-strand DNA antibodies >800 IU/mL (normal <35 IU/mL). Antiphospholipid antibodies were negative. Direct Coombs test was positive; 24-h urine albumin was 1156 mg/day. A diagnosis of IPO secondary to SLE was made; intravenous pulse methylprednisolone was administered in view of deranged renal functions. In view of oliguria with worsening renal functions, hemodialysis was started. She continued to have pain in abdomen and copious aspirates for 10 days after institution of steroids. In view of persistent GI symptoms and renal failure, she also received pulse cyclophosphamide and one dose of intravenous immunoglobulin (IVIG) (1g/kg/day for 2 days). After initiating immunosuppression, she showed decrease in Ryle's tube aspirates, abatement of abdominal pain, and decrease in abdominal girth. She was gradually started on liquid diet and shifted to full feeds. Renal recovery took 2 weeks. Renal biopsy post stabilization revealed one sclerosis and 20 viable glomeruli showing thin basement membranes and patent capillary lumina. Few glomeruli showed mild proliferation of mesangial cells with tiny bands of fibrous tissue in the interstitium. Immunofluorescence showed IgG and C3 faintly positive in the mesangium, while IgM, IgA, C1q, kappa, and lambda were negative probably as the patient had already received immunosuppression. Electron microscopy showed diffuse foot process flattening, plenty of subepithelial and mesangial electron-dense deposits, and presence of tubulo-reticular body [Figure 2]. The histopathologic diagnosis was membranous lupus nephritis class V according to the International Society of Nephrology (ISN)/Renal Pathology Society (RPS).

- X-ray abdomen showing multiple air fluid levels
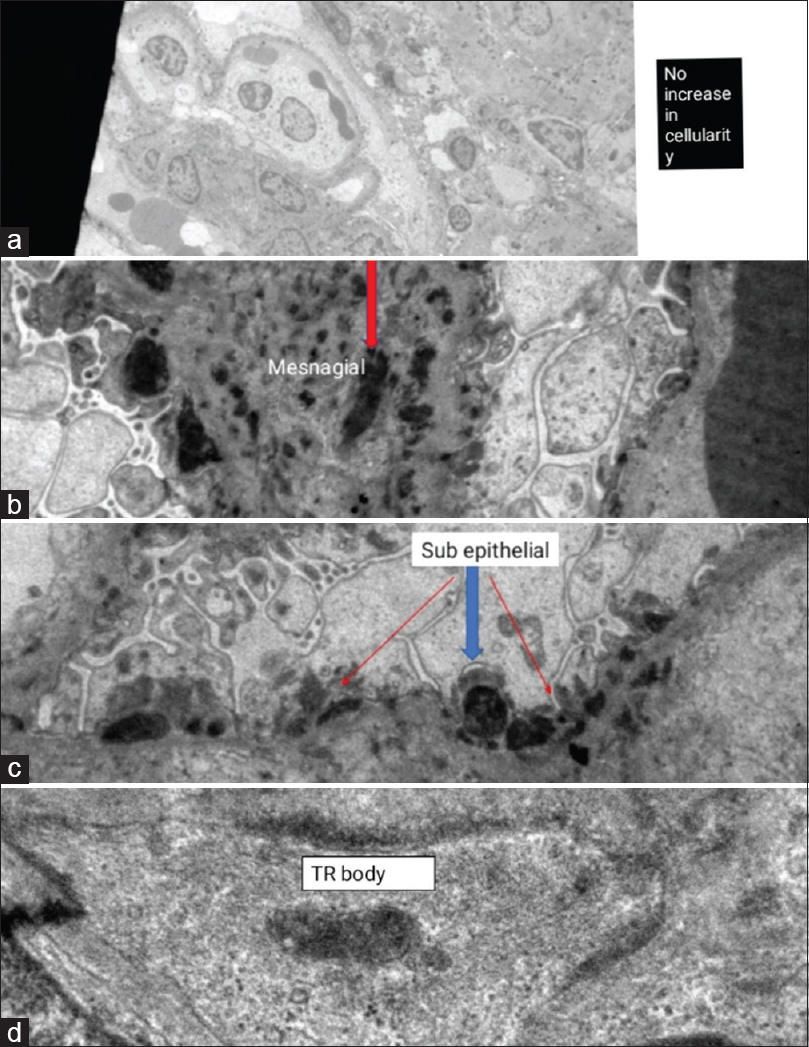
- Electron microscopy showing (a) no increase in cellularity (total magnification 2.00kx), (b) subepithelial electron-dense deposits (total magnification 8.00kx), (c) mesangial electron-dense deposits (total magnification 10.00kx), and (d) tubulo-reticular body (total magnification 50.00kx)
She was discharged on full oral feeds, full-dose daily steroids, and six doses of fortnightly cyclophosphamide followed by mycophenolate mofetil as maintenance immunosuppression. At follow-up after 1 year, she had no recurrence of GI symptoms or evidence of renal flare in terms of proteinuria, hematuria, or hypertension, maintained normal renal functions, and exhibited good weight gain.
Discussion
The diagnosis of IPO rests on the presence of features of intestinal obstruction in the absence of a demonstrable anatomic or neurologic cause.[1] The symptoms of IPO include acute or subacute onset of abdominal pain, nausea, vomiting, abdominal distension, constipation, diarrhea, and weight loss.[3] IPO can be classified as acute when the onset of one or more symptoms of IPO is less than 6 months before the diagnosis or chronic when the symptoms are present for more than 6 months.[3456] IPO may occur secondary to an underlying disorder affecting the neuromuscular function, like SLE, systemic sclerosis, or amyloidosis, use of drugs such as opiates, previous surgery, or may rarely occur as a primary illness.[567] We excluded drugs, previous surgery, and an underlying neurological disease based on the medical history, examination, and relevant investigations. Multisystem involvement in the form of serositis, abnormal hematologic parameters, and renal dysfunction coupled with strongly positive serology suggested GI features of IPO secondary to SLE. Till date, IPO has been considered a very rare complication of lupus. In a recent series of hospitalized patients with SLE, the prevalence of lupus IPO (SLE-IPO) was reported to be approximately 2%. In this series, SLE-IPO encompassed about 5% of all cases of SLE-induced abdominal pain requiring admission to the hospital.[8] Among the reported cases of SLE-IPO, majority were females of Asian origin and had undergone laparotomy before diagnosis of IPO was recognized. In many cases, it occurred as a multisystem disease, although rarely IPO has been reported as a sole presentation of lupus. Patients may present symptoms that precede the diagnosis of lupus from 11 to 66 days and even up to 2 years; such cases pose a great diagnostic challenge to both clinicians and surgeons.[5] The pathophysiology of IPO with SLE remains unclear, but several hypotheses have been proposed. One study by Perlemuter et al. suggests that a generalized vasculitis involving inflammatory fibrinoid deposits affects the smooth muscle of the intestine leading to small bowel obstruction; another proposed mechanism is smooth muscle dysmotility affecting the muscularis propria.[6] There is an apparent association between IPO related to SLE and urological manifestations, especially uretero-hydronephrosis, which was present in 59.53%–66.7% of cases in different case series, but not in our case.[7] The definitive diagnosis of IPO is based on the signs and symptoms of intestinal obstruction and evidence of intestinal obstruction in the abdominal X-ray and CT images, without any evidence of anatomical or structural abnormalities.[91011] Functional studies, such as antro-duodenal manometry, which show intestinal hypomotility and esophageal aperistalsis, may be performed when there is ambiguity in diagnosis of primary IPO.[11] Treatment should be individualized and generally multidisciplinary. Among the recommendations for management of SLE issued by the European League against Rheumatism and established by the American College of Rheumatology (ACR), there is no separate scheme suggested for GI manifestations, and so, immunosuppressive protocol of lupus nephritis was followed.[12] Some clinicians have tried erythromycin because of its dual antimicrobial and prokinetic properties with variable results.[1011] Surgery has produced disappointing results with relapses within a few months, incidence of GI bleeding, and fungal peritonitis.[1314] Most gratifying results have been reported with conservative management of intestinal obstruction and use of parenteral nutrition and immunosuppressive therapy. A case report by Lopez et al. mentioned use of IVIG with a good clinical response if the patient is refractory to steroids, and hence was used in our case.[15] The long-term outcomes of SLE-related IPO have been variable. Most cases do well with ongoing maintenance immunosuppressive therapy, but some patients developed recurrent attacks of IPO without involvement of other organs. The mortality of this complication of SLE can be up to 23.52%, most often due to development of fungal peritonitis and GI bleeding.[1314]
Conclusion
IPO secondary to lupus is an under-recognized disorder that may initially manifest together with other organ system involvement or may precede other system involvement by many months. Management of the disorder should include early suspicion, timely diagnosis, and appropriate management of underlying secondary disorder with corticosteroids, immunosuppressants, and other immunomodulators like IVIG.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Dr. Shaila Khubchandani is acknowledged for providing figures of electron microscopy.
References
- Intestinal pseudo-obstruction as a presenting manifestation of systemic lupus erythematosus: Case report and review of the literature. South Med J. 2004;97:186-9.
- [Google Scholar]
- Systemic lupus erythematosus: Demographics, prognosis, and outcome. J Rheumatol Suppl. 1997;48:67-71.
- [Google Scholar]
- Clinical features, morbidity, and risk factors of intestinal pseudo-obstruction in systemic lupus erythematosus: A retrospective case control study. J Rheumatol. 2016;43:559-64.
- [Google Scholar]
- Intestinal pseudo-obstruction syndrome in systemic lupus erythematosus. Lupus. 2011;20:1324-8.
- [Google Scholar]
- Chronic intestinal pseudo-obstruction in patients with systemic lupus erythematosus: Report of four cases. Clin Rheumatol. 2008;27:399-402.
- [Google Scholar]
- Chronic intestinal pseudo-obstruction in systemic lupus erythematosus. Gut. 1998;43:117-22.
- [Google Scholar]
- Intestinal pseudo-obstruction in systemic lupus erythematosus: An uncommon but important clinical manifestation. Lupus. 2000;9:11-8.
- [Google Scholar]
- Clinical features and associated factors of abdominal pain in systemic lupus erythematosus. J Rheumatoid. 2013;40:2015-22.
- [Google Scholar]
- A review of gastrointestinal manifestations of systemic lupus erythematosus. Rheumatology (Oxford). 1999;38:917-22.
- [Google Scholar]
- Gastrointestinal and hepatic manifestations of systemic lupus erythematosus. J Clin Gastroenterol. 2011;45:436-41.
- [Google Scholar]
- Gastrointestinal involvement in systemic lupus erythematosus: Insight into pathogenesis, diagnosis and treatment. World J Gastroenterol. 2010;16:2971-7.
- [Google Scholar]
- Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-86.
- [Google Scholar]
- Association of paralytic ileus, intestinal cystitis and hydronephrosis with systemic lupus erythematosus. Hong Kong Med J. 1995;1:354-6.
- [Google Scholar]
- Successful treatment with intravenous immunoglobulin's in a patient with intestinal pseudo-obstruction associated with systemic lupus erythematosus. Rev ColombRheumatol. 2017;24:123-8.
- [Google Scholar]







