Translate this page into:
Ataxia in a chronic kidney disease patient on anti-tubercular therapy
Address for correspondence: Dr. M. K. Phanish, Department of Nephrology and Renal Transplantation, Medanta, The Medicity, Gurgaon, Haryana, India. E-mail: phanishmk@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Isoniazid is the mainstay of anti-tubercular therapy. Used in isolation or in combination with other anti-tubercular drugs, it is generally well-tolerated. While hepatotoxicity and neurotoxicity are reported, significant neurotoxicity remains uncommon. In this report, we present a case of rare neurological complication secondary to anti-tubercular therapy in a patient with stage 5 chronic kidney disease.
Keywords
Ataxia
cerebellum
chronic kidney disease
dentate nuclei
isoniazid toxicity
Introduction
Isoniazid is widely used for the treatment of tuberculosis. Neurotoxicity due to Isoniazid generally manifests as peripheral neuropathy. In this report we present a patient with stage5 CKD who developed rare central nervous system toxicity due to Isoniazid.
Case Report
A 29-year-old woman, a known case of chronic kidney disease (CKD) presented with history of fever from last 4 months. Her basic renal disease was unknown as she had presented with bilateral small kidneys. Fever was high grade, intermittent and was associated with poor appetite. She had no dysuria, cough or abdominal complaints. The examination was unremarkable except for left-sided axillary lymphadenopathy. There was no hepatosplenomegaly and breast examination was normal. Neurological examination was unremarkable. Her creatinine on admission was 6.9 mg/dl with an estimated glomerular filtration rate (eGFR) of 7 ml/min/1.73 m2.
She was evaluated for fever. Serological tests for malaria, dengue, and enteric fever were negative; urine cultures were sterile. Her hemoglobin, total leukocyte count and platelet counts were 8.5 g/dl, 5880/µl, and 1.5 lakhs/µl, respectively. The kidney function tests revealed serum urea of 223 mg/dl and serum creatinine of 6.90 mg/dl. The serum sodium and potassium levels were 136 mmol/l and 4.8 mmol/l, respectively. The liver function tests and thyroid function tests were normal. Blood cultures were sterile.
Ultrasound abdomen showed hepatosplenomegaly with irregular, shrunken kidneys. There was no hydroureteronephrosis. Computed tomography of chest and abdomen showed splenomegaly, retroperitoneal and axillary lymphadenopathy with no mediastinal lymphadenopathy. There was no consolidation, collapse or cavitating lesion.
Fine needle aspiration from the axillary lymph node was inconclusive. Endoscopic ultrasound-guided fine needle aspiration cytology of the retroperitoneal nodes showed reactive lymphoid cells with few histiocytes. Her tuberculosis gold quantiferon test was positive. In view of persistent fever, lymphadenopathy, hepatosplenomegaly and positive quantiferon gold test, she was empirically initiated on antitubercular therapy with four drugs (isoniazid [INH], rifampicin, pyrazinamide and ethambutol) with appropriate dose adjustments for her kidney function. She was also commenced on pyridoxine 40 mg once daily. Hemodialysis (HD) was initiated through left radiocephalic fistula. One-week after initiation of antitubercular therapy, she developed slurring of speech with unstable gait. On examination, she had pulse 100 beats/min, blood pressure was 150/90 mmHg with no postural drop. The oxygen saturations were 100% on room air, blood glucose levels, serum sodium, and calcium levels were normal. Central nervous system examination showed dysarthria with impaired finger-nose test on left side with past pointing. Her gait was ataxic with swaying on left side. She was unable to perform rapid alternating movements on left side. Motor examination done showed decreased tone with normal power and reflexes on left side. There were no neurological findings on the right side. Sensory examination was normal with negative Romberg's sign.
Magnetic resonance imaging (MRI) brain showed diffusion restriction in bilateral dentate nuclei with T2 fluid-attenuated inversion recovery hyperintensity with no evidence of hemorrhage [Figure 1]. There were no features suggestive of tuberculoma or abscess. As there was no other obvious explanation of the sudden development of cerebellar ataxia, a diagnosis of INH induced toxic encephalopathy was considered and INH was withdrawn. Two days after withdrawal of INH, patient's condition started to improve and had complete neurological recovery within a week. She was commenced on HD via a left radio-cephalic arteriovenous fistula. She was discharged on modified antitubercular therapy that included rifampicin at dose of 450 mg/day, pyrazinamide at dose of 500 mg twice daily and levofloxacin at dose of 250 mg/day. On follow-up after 3 weeks, she has remained afebrile with no neurodeficit.
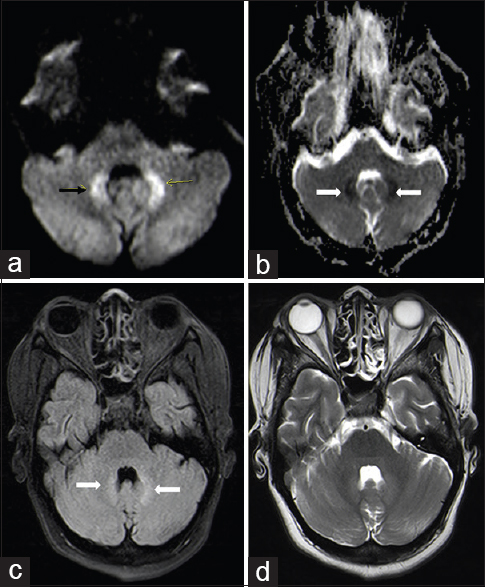
- Diffusion-weighted image, (a) diffusion restriction in bilateral dentate (arrow) nucleus in cerebellum showing corresponding low apparent diffusion coefficient (ADC) value on ADC images (b), hyperintensity noted on T2 fluid-attenuated inversion recovery images (c). There are no signal changes seen on T2 weighted images (d)
Discussion
Isoniazid is a bactericidal antitubercular drug that interferes with pyridoxine metabolism leading to deficiency of this vitamin. Adverse effects occur in about 5% of patients on INH, who are also on adequate doses of pyridoxine (10–50 mg/day). Common neurotoxicity of INH is that of peripheral neuropathy that is usually mild and reversible. Due to its hepatic clearance, no dose modification is generally required in patients with kidney disease. INH is used extensively in patients with CKD, on dialysis and following a renal transplant.[1] It is usually well-tolerated in this population and when toxicity occurs, it generally manifests with hepatotoxicity.
Central nervous system toxicity due to INH presenting with altered consciousness level secondary to encephalopathy has been described, but cerebellar ataxia is very rare.[2] Severe neurotoxicity presenting with encephalopathy and seizures has been described with INH overdose in children which requires treatment with intravenous pyridoxine.[3] Our patient was given with 40 mg/day of pyridoxine along with INH 300 mg once a day. She was not treated with higher doses of pyridoxine as the neurological features were not severe, and they responded to withdrawal of the drug. Cerebellar ataxia due to INH has been described in the past in children[4] but to our knowledge, it has not been described before in a patient with stage 5 CKD with MRI scan demonstrating hyperintense lesions in the dentate nuclei. However, it is worthwhile noting that cerebellar lesions with similar involvement of dentate nuclei have been described before in a patient on metronidazole therapy.[5] Our patient was not taking metronidazole. We did not measure INH levels in our patient and therefore we are unable to comment if the toxicity was related to drug levels.
In summary, we describe a patient with stage 5 CKD developing cerebellar ataxia and MRI findings of B/L dentate nuclei lesions after the commencement of INH. The neurological features completely resolved after withdrawal of INH. Clinicians should be aware of this rare complication of INH therapy.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Prospective randomized control trial of isoniazid chemoprophylaxis during renal replacement therapy. Transpl Infect Dis. 2005;7:99-108.
- [Google Scholar]
- Encephalopathy caused by isoniazid in a patient with end stage renal disease with extrapulmonary tuberculosis. Ren Fail. 2003;25:135-8.
- [Google Scholar]
- Abnormal enhancing lesion of dentate nuclei causing neurologic symptoms induced by metronidazole toxicity. Clin Gastroenterol Hepatol. 2005;3:xxix.
- [Google Scholar]







