Translate this page into:
Atheroembolic Renal Disease: A Case Series
Address for correspondence: Dr. Anila A Kurien, Renopath, Center for Renal and Urological Pathology, No 27 and 28, VMT Nagar, Kolathur, Chennai, Tamil Nadu - 600 099, India. E-mail: anila_abraham08@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Atheroembolic renal disease (AERD), a part of systemic cholesterol embolization syndrome, is caused by the occlusion of small arteries in the kidneys by cholesterol crystal emboli from ulcerated atherosclerotic plaques. Kidney is commonly involved because of its proximity to the abdominal aorta and its enormous blood supply. AERD is an under diagnosed condition. We report eight cases of AERD, highlighting the variability in its clinical presentation and the importance of a renal biopsy to arrive at a definitive diagnosis.
Keywords
Atheroembolism
cholesterol clefts
kidney biopsy
Introduction
Atheroembolic renal disease (AERD) is defined as a renal failure occurring secondary to renal artery, arterioles, or glomerular capillary occlusion by atheromatous plaque dislodged from major arteries. It may occur following intravascular interventions, or rarely, can occur spontaneously also.[1] We present eight cases of AERD reported in our institute among 15,417 native kidney biopsies received from August 2013 to June 2018.
Case Reports
Case 1
In September 2013, a 66–year-old hypertensive male patient presented with severe chest pain and breathlessness. His serum creatinine was 1.5 mg/dl. He was diagnosed to have anterior wall myocardial infarction. He was thrombolyzed with streptokinase. Two months later, he developed increased shortness of breath and was hospitalized again. He was found to have dialysis requiring renal failure. At the time of admission, his blood urea was 158 mg/dl, serum creatinine was 8 mg/dl, ESR-35/70 mm/hr. His C3 was 86.2 mg/dl and C4 30.6 mg/dl. His renal biopsy revealed atheroemboli in an arteriole.
Case 2
A 48-year-old male patient, a known hypertensive and diabetic, was admitted in August 2014, with a serum creatinine of 6.9 mg/dl and 3+ proteinuria. He was diagnosed as having AERD by kidney biopsy. There was no predisposing factor for AERD in this patient.
Case 3
A 55-year-old male patient presented with azotemia and subnephrotic proteinuria with a serum creatinine of 3.2 mg/dl and protein creatinine ratio of 1.1 mg/dl in July 2015. He was a known hypertensive. Ultrasound showed normal sized kidneys with increased echoes. His renal biopsy revealed AERD. There was no history of prior invasive procedures.
Case 4
A 56-year-old female patient, a known hypertensive, was admitted in March 2016 with a serum creatinine of 5.7 mg/dl and 2+ proteinuria. She underwent hemodialysis. Renal biopsy showed cholesterol emboli in an interlobular artery.
Case 5
A 64–year-old male patient, a known hypertensive, developed coronary artery disease in January 2015. He had severe left ventricular dysfunction with ejection fraction of 38%. His serum creatinine was 0.8 mg/dl. He was started on aspirin, clopidogrel, furosemide, and digoxin. In January 2017, he was admitted with acute pulmonary edema and left little toe gangrene. His creatinine was 4.2 mg/dl. He underwent four cycles of hemodialysis. Renal artery doppler findings were suggestive of right renal parenchymal disease and left renal artery stenosis. Renal biopsy revealed AERD.
Case 6
In May 2017, a 73-year-old male patient developed breathlessness. His echocardiography study showed global hypokinesia and ejection fraction of 45%. Cardiac angiogram was done and was started on aspirin. His creatinine increased from 1.7 mg/dl to 9.6 mg/dl. He was started on hemodialysis. He was diagnosed as having AERD by renal biopsy.
Case 7
A 63-year-old male patient, a known diabetic and hypertensive, underwent kidney biopsy in December 2017 for renal failure of unknown cause with serum creatinine of 2.1 mg/dl and was found to have AERD. He underwent coronary artery bypass grafting (CABG) on 26th January 2018.
Case 8
A 62-year-old male patient, a known diabetic and hypertensive, developed flash pulmonary edema, underwent coronary and renal angiography. He was found to have left renal artery stenosis and underwent percutaneous transluminal angioplasty. Three weeks later, he developed acute kidney injury. Renal biopsy was done and atheroemboli was identified in an arteriole.
Discussion
In cholesterol embolization syndrome, there occurs arterio-arterial embolization of cholesterol crystals from an atheromatous plaque located within proximal large arteries to distal small arteries and arterioles. Cholesterol crystals tend to lodge in small arteries that are 150--200 μm in diameter. They are most commonly seen at the bifurcations. The organs involved are kidney, skin, gastrointestinal tract, central nervous system, muscle, eyes, and extremities.[2]
Panum was the first to describe AERD in the year 1862.[3] It can cause acute, subacute, or chronic renal failure as follows: (a) following massive embolization, acute renal failure develops in a few days, (b) subacute form of renal failure occurs as a result of recurrent embolization, (c) in chronic AERD, cholesterol crystals are slowly released from the eroded atherosclerotic plaque over a period of time.[4]
Risk factors
AERD may occur spontaneously or after invasive procedures in the heart and vasculature (iatrogenic AERD) such as coronary angiography, aortic angiography, cardiac catheterization, and cardiovascular surgery. Vascular surgery has the inherent risk of disrupting the plaques during clamping, incision, or while manipulating the vessels. Radiological instrumentation of aorta can cause mechanical trauma.[5]
AERD can also occur following thrombolysis or anticoagulation. Thrombolytic agents can lyse thrombi covering atherosclerotic plaques, thereby releasing cholesterol crystals into the circulation. Heparin and oral anticoagulants may lead onto hemorrhage inside a complex plaque and can initiate its disruption. They may also prevent protective thrombus formation over an ulcerated plaque.[6] Cardio pulmonary resuscitation may also lead onto AERD. Predisposing factors such as coronary angiogram or thrombolytics were present in three of our patients (37.5%).
Plaques with thin fibrous cap and abundant extracellular lipid rich core are prone to rupture.[7] The resulting mechanical obstruction causes end organ damage by tissue ischemia and necrosis (necroinflammation).[4] Following obstruction, neutrophilic and eosinophilic infiltration occurs and they secrete various inflammatory and vasospastic mediators. This is followed by mononuclear cell accumulation. Often giant cells are also seen. Hyperplasia of intima and perivascular fibrosis occur later on which leads to further obstruction.[8] Although recanalization may occur in some vessels, one may still find the cholesterol crystals within the lumen of the affected vessel. Ongoing ischemic damage leads onto varying degrees of glomerulosclerosis, interstitial fibrosis, and tubular atrophy. Areas downstream of the occlusion show only nonspecific ischemic changes.
Diagnosis
AERD was most often diagnosed postmortem in the past. However, increasing awareness about this condition helps us to make a correct premortem diagnosis.[5] AERD can present with many nonspecific signs and symptoms. It mimics other conditions that cause renal failure. Even when suspected, it is difficult to make the diagnosis of AERD.[9] Typically male patients older than 60 years, with a history of smoking and hypertension, are affected.[10] In such a patient, classical triad of a precipitating factor, skin lesions, and acute or subacute renal failure is highly suggestive of AERD.[8] In our study, among the eight patients, seven (87.5%) were male patients. Age range was from 48--73 years. Mean age was 60.14 years. In only one patient (12.5%), AERD was suspected at the time of renal biopsy. Seven patients (87.5%) were known hypertensives. Three patients were known diabetic (37.5%) and four patients (50%) were chronic smokers.
Laboratory findings
The laboratory findings include C3 hypocomplementemia,[11] increased serum cholesterol, C-reactive protein, and erythrocyte sedimentation rate. Activated T lymphocytes secrete interleukin 5, which in turn leads onto transient eosinophilia.[12] Sometimes, eosinophiluria also occurs. Eosinophilia was present in three of our patients (37.5%). Serum cholesterol was elevated in all the eight patients and high ESR was seen in six patients (75%).
Renal biopsy
Renal biopsy is the gold standard to diagnose AERD.[13] Because the renal vasculature is not uniformly affected, the diagnosis may be missed if the affected area is not included in the biopsy. Within the affected area if cholesterol clefts are not seen, then the diagnosis is not possible. When AERD is suspected, it is essential to examine the entire biopsied material.[9] The sensitivity of renal biopsy in diagnosing AERD is 75%. However, when two biopsies are taken from the same patient, the sensitivity increases to 94%.[1] Needle shaped, biconvex clefts are seen in the arcuate and interlobular arteries, as the lipids are dissolved during fixation. Surrounding the cleft-like space, foreign body type giant cells may be seen. Cholesterol emboli can rarely lodge in the afferent arteriole and in the glomeruli.[14] With complete obstruction, distal infarction and necrosis occurs. Usually, the obstruction is incomplete resulting in ischemic atrophy. When unfixed, unstained tissue is air dried and viewed under polarized light, cholesterol crystals are birefringent.[1] Since many of these patients are hypertensives, arteriolar nephrosclerosis is also a common finding.[15]
In our study, needle shaped biconvex clefts, surrounded by intimal fibrosis, were identified in interlobular arteries in five patients and in arterioles in three patients [Figures 1-3]. The mean percentage of globally sclerotic glomeruli was 49.54%. Among the eight patients, mild IFTA was present in two (25%), moderate in five (62.5%), and severe in one (12.5%) patient. Findings of immunofluorescence study were noncontributory in our patients. Electron microscopic study was not done in any of these patients.
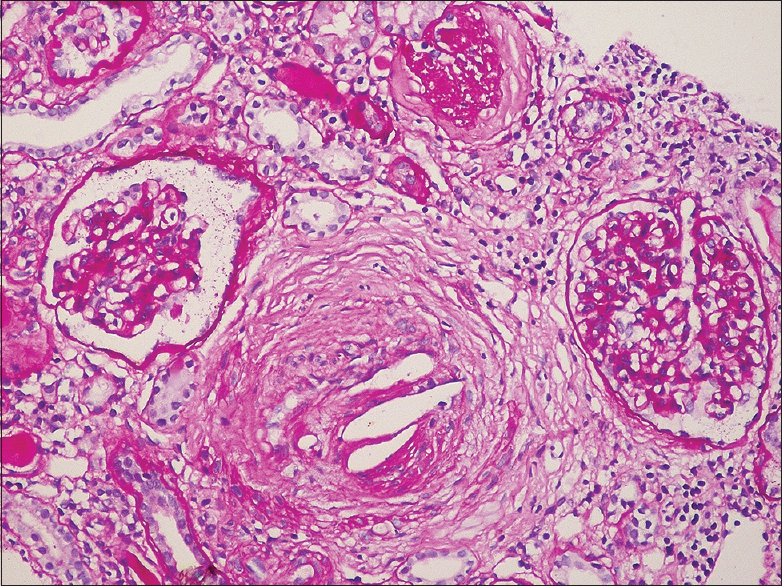
- The interlobular artery is occluded by cholesterol emboli, with cleft like spaces surrounded by a fibrous reaction. The adjacent cortex shows interstitial fibrosis, tubular atrophy and ischemic glomerular changes. Periodic acid Schiff × 200

- Cholesterol emboli within a small interlobular artery. Macrophages surround the crystals. Hematoxylin and eosin × 200

- Cholesterol crystal occludes the lumen of an artery which also shows fibrous intimal proliferation and duplication of internal elastic lamina which are features of underlying hypertensive arterionephrosclerosis. Jones methenamine silver × 200
Treatment
Although a specific treatment is not available, a correct diagnosis can save the patient from inappropriate treatment.[5] Studies have shown that the use of statins is beneficial. Statins help in plaque stabilization by its anti-inflammatory and lipid lowering properties.[10] Except one, all of our patients received statins [Table 1]. Steroids decrease the inflammatory response accompanying AERD.[8]
| Case No | Treatment | Serum creatinine at the time of biopsy (mg/dl) | Outcome |
|---|---|---|---|
| 1 | Statins, steroids and hemodialysis | 8 | He expired after one year |
| 2 | Statins and hemodialysis | 6.9 | Lost to follow up |
| 3 | Statins. Not on dialysis | 3.2 | Renal function improved, last follow up creatinine -1.6mg/dl (August 2018) |
| 4 | Steroids and hemodialysis | 5.7 | Renal function improved, last follow up creatinine-1.8mg/dl (January 2017) |
| 5 | Statins and hemodialysis | 4.2 | Died of septic shock on 29.3.17 |
| 6 | Statins, steroid and hemodialysis | 9.6 | As on 9.8.18 continues to be dialysis dependent |
| 7 | Statins. Not on dialysis. | 2.1 | As on 22.2.18, creatinine -1.7mg/dl and proteinuria 1+ |
| 8 | Statins and hemodialysis | 9 | Follow up details not available |
Prognosis
Mortality in AERD occurs most commonly due to the underlying cardiovascular disease. The prognosis of iatrogenic AERD is worse than spontaneous AERD.[10] Among our eight patients, two patients succumbed to the underlying disease. One patient continues to be dialysis dependent. Renal function improved in three patients and one patient was lost to follow-up. Follow-up details are not available in one patient [Table 1].
Conclusion
Early diagnosis is imperative to prevent further showers of cholesterol emboli. AERD should be considered as a differential diagnosis in hypertensive patients who present with progressive renal failure of unknown etiology. The absence of recent vascular interventions, anticoagulant/thrombolytic therapy, or the lack of characteristic clinical presentation should not discourage the clinician from considering AERD in these patients. Our study also highlights the importance of performing a kidney biopsy to make a diagnosis of AERD.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cholesterol crystal embolization: Areview of 221 cases in the English literature. Angiology. 1987;42:769-84.
- [Google Scholar]
- Experimentelle Beitrage zur Lehre von der embolie. Virchows Arch PatholAnatPhysiol. 1862;25:308-10.
- [Google Scholar]
- Cholesterol crystal embolism: Arecognizable cause of renal disease. Am J Kidney Dis. 2000;36:1089-109.
- [Google Scholar]
- Cholesterol crystal embolization caused by anticoagulant therapy. Int J Dermatol. 2009;48:989-90.
- [Google Scholar]
- Renal atheroembolic disease: The cinderella of nephrology? Nephrol Dial Transplant. 1996;11:1516-7.
- [Google Scholar]
- The challenge of diagnosing atheroembolic renal disease: Clinical features and prognostic factors. Circulation. 2007;116:298-304.
- [Google Scholar]
- Atheroembolic renal disease causes hypocomplementaemia (abstract) Lancet. 1985;2:118-21.
- [Google Scholar]
- Eosinophilia as a clue to the diagnosis of atheroembolic renal disease. Arch Intern Med. 1987;147:1384-5.
- [Google Scholar]
- Favorable outcome in atheroembolic renal disease with pulse steroid therapy. Indian J Nephro l. 2012;22:473-6.
- [Google Scholar]
- Arterial occlusion produced by emboli from eroded aortic atheromatous plaques. Am J Pathol. 1945;21:549-65.
- [Google Scholar]
- Histopathologic evaluation of atheroembolic renal disease (AERD) [Abstract] J Am Soc Nephrol. 1997;8:534A.
- [Google Scholar]







