Translate this page into:
Atypical presentation of post infectious glomerulonephritis as malignant hypertension and thrombotic microangiopathy
Address for correspondence: Dr. Mahesha Vankalakunti, Department of Pathology and Laboratory Medicine, Manipal Hospital, #98, Rustom Bagh, HAL Airport Road, Bangalore - 560 017, Karnataka, India. E-mail: vkmahesh123@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Infection-related glomerulonephritis presents commonly as acute nephritic illness, hypertension, hypocomplementinemia following an episode of pharyngitis or pyoderma. Atypical features like thrombotic microangiopathy (TMA), produced by neuraminidase antigen targeting endothelium have been described rarely. We report a case of TMA secondary to malignant hypertension, coexisting with post infectious glomerulonephritis.
Keywords
Kidney biopsy
malignant hypertension
post infectious glomerulonephritis
thrombotic microangiopathy
Introduction
Acute post infectious glomerulonephritis (APIGN) is defined as acute nephritic illness preceded by pharyngitis or pyoderma.[12] Atypical features like thrombotic microangiopathy (TMA) are rarely described in association with APIGN. Renal manifestations of TMA range from asymptomatic urinary abnormalities to fulminant oligoanuric renal insufficiency. Neuraminidase antigen from streptococcal infection is said to produce endothelial injury and cause TMA.[34567] We report a case of APIGN with focal crescents associated with TMA secondary to malignant hypertension. Altered renal parameters were restored to normal after successful bllod pressure control and steroids therapy.
Case Report
A 28-year-old male was healthy until one month before admission, when he experienced increasing tiredness and lethargy. Two days before hospitalization, he developed fever, headache with blurring of vision. On the day of admission he had one episode of convulsions and developedaltered sensorium. Clinical examination revealed pallor bilateral pitting pedal edema. Blood pressure was 180/100 mmHg in supine position; heart rate, 96 beats/min; temperature, 101° F; respiratory rate, 32 breaths/min. Neurologic examination suggested postictal confusion with papilloedema, bilateral flexor plantar reflex and no signs of neck stiffness. Lungs showed bibasal rales. Rest of the physical examination was unremarkable. Initial laboratory investigation showed serum creatinine, 3.89 mg/dl; blood urea nitrogen, 84 mg/dl; serum potassium, 4.5 mEq/l; chloride, 99 mmol/l; sodium, 134 mmol/l; white blood cell count, 13600 cells/cumm; hemoglobin, 7.8 gm/dl; platelet count, 90000/cumm; calcium, 8.6 mg/dl; phosphorus, 3.5 mg/dl; albumin, 3.6 gm/dl; bilirubin 0.9 mg/dl; serum iron, 20 μmol/l and lactate dehydrogenase(LDH), 1280 U/l. Urinanalysis showed specific gravity 1.025, pH 6.0, 4+ protein, 15-20 pus cells/hpf, 25-30 red blood cells (RBCs)/hpf and 1-2 granular casts. 24 h urine protein leak was 1.9 g/d. Liver function test, lipid profile and thyroid function tests were within normal limits. On ultrasonography - both kidneys were of normal size, but with increased parenchymal echogenicity. Computed tomography scan of head revealed ill-defined hypodensities at bilateral parieto-occipital region consistent with posterior reversible encephalopathy syndrome. Because of the complex clinical syndrome (hypertensive encephalopathy syndrome coupled with proteinuria and active urine sediments), a percutaneous renal biopsy was performed. Serologic tests were negative for anti-streptolysin-O antibody titre, antinuclear antibody, anti-double stranded deoxyribonucleic acid antibody, anti-neutrophil cytoplasmic antibodies profile by ELISA and anti-glomerular basement membrane antibody. Serum complement levels (C3, C4) were normal. Screening for hepatitis B, hepatitis C and human immunodeficiency virus was negative. On light microscopy, there were twenty two viable glomeruli displaying two pathologies simultaneously. Majority of them appeared solid and proliferative comprising neutrophilic infiltration in the capillary tufts obliterating capillary lumen secondary to proliferation of endothelial cells, neutrophils and sparse mesangial cells; with single contoured basement membranes [Figure 1]. Two of these glomeruli (9%) possessed circumferential oriented active cellular crescents, without necrotizing lesions [Figure 2]. Lobular accentuation was absent. In addition, five glomeruli (devoid of neutrophilic infiltration) showed changes of TMS in the form of blood filled, engorged capillaries [Figure 3], and fibrin thrombus in the hilar arteriole. Mesangiolysis was noted. Moderate degree of acute tubular injury was noticed with fresh RBC's in the lumen. There was no focus of tubular atrophy/interstitial fibrosis. Interstitium lacked inflammation or granuloma. Artery and arterioles showed hypertensive changes to a moderate extent in the form of hypertrophic muscular wall and intimal thickening. Marked intimal hyalinosis was present in smaller arterioles. Fibrinoid necrosis of vessel wall, luminal thrombosis or vasculitis was not evident. Immunofluorescence revealed diffuse and global, coarse granular deposits along the capillary walls and mesangium with C3 (3+) and IgG (2+) in five available glomeruli [Figure 4]. Kappa and lambda light chain stains showed 2+ positivity in a similar pattern. C3 deposits were also seen in vessels. IgA, IgM and C1q were negative. A diagnosis of coexistent TMA and APIGN with focal (9%) crescents was established. Upon probing, patient denied any symptom of previous infective episode.

- Solid and proliferative tufts comprising neutrophilic infiltration, obliterating capillary lumen. Basement membranes are single contoured (periodic acid Schiff, ×40)
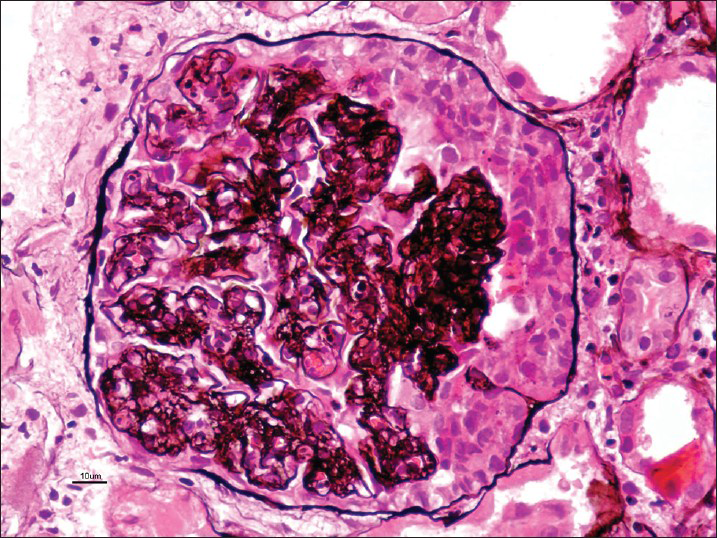
- Globally proliferative tuft with circumferential active crescent. Basement membranes are single contoured (Periodic Schiff-Methenamine Silver, ×40)
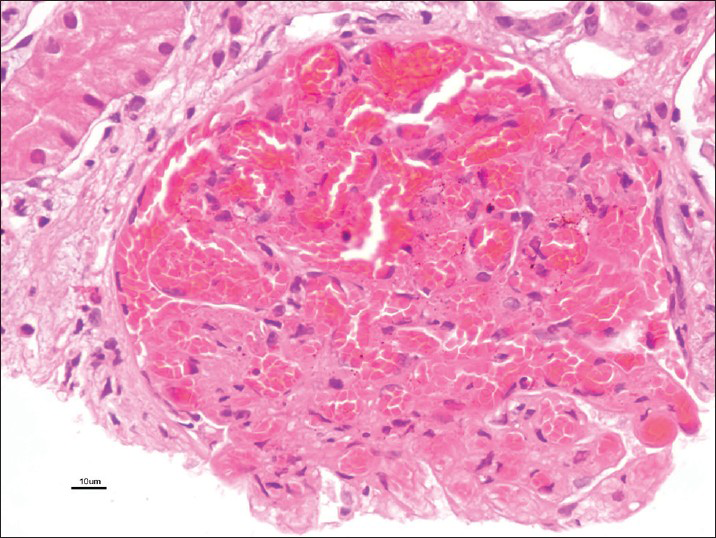
- Glomerulus revealing blood filled and engorged capillaries with mesangiolysis (H and E, ×40)
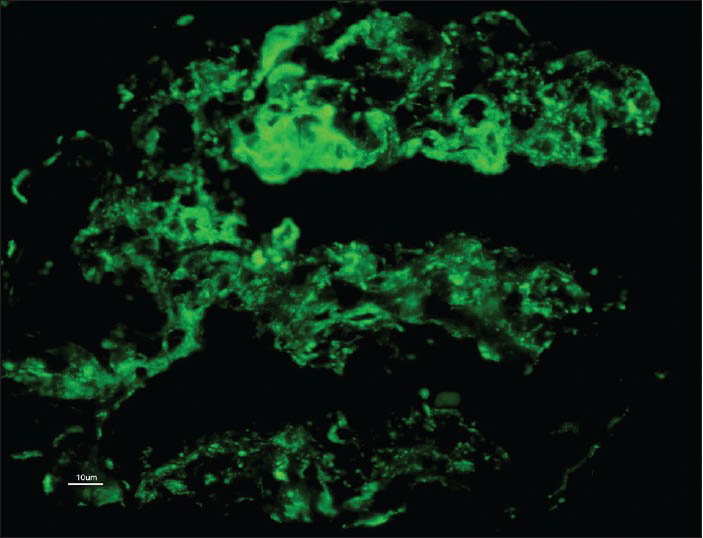
- Diffuse and global, coarse granular deposits along the capillary walls and mesangium with C3 (3+)
Patient was treated with intravenous antibiotics, intravenous pulse methyl prednisolone (1 g for 3 days) considering the presence of active crescents histologically, followed by tapering dose of oral prednisolone; along with strict control of blood pressure. He was treated with intravenous nitroglycerine infusion during the early phase of the disease, followed by oral ramipril 5 mg twice daily, clonidine 0.1 mg thrice daily, frusemide 40 mg thrice daily, metalozone 5 mg twice daily and prazocin 5 mg twice daily. There was a dramatic improvement in his renal function with decrease in serum creatinine (1.5 mg/dl) as well as trace proteinuria. TMA was also resolved as evidenced by normalization of serum LDH level, hemoglobin and platelet count.
Discussion
The clinical presentation of APIGN varies from asymptomatic, microscopic hematuria to full blown acute nephritic syndrome.[189] Renal biopsy is generally not indicated, except in atypical situations. Biopsy incidence in adults varies from 0.6% to 4.6%.[9] Histologically, it appears most commonly as acute endocapillary proliferative glomerulonephritis than mesangial proliferative glomerulonephritis (GN).[910] Less commonly, it appears as focal or diffuse crescentic GN. Hypertension is present in 50-90% of patients and varies from mild to severe. It is primarily caused by fluid retention. Malignant hypertension leading to TMA is an uncommon but serious complication. Multiple studies have shown that majority of children with epidemic or nonepidemic form APIGN has excellent prognosis. Recent data on outcome of APIGN in adults from North American and European study group shows that: 28-64% had complete remission; 27-53% had persistent renal dysfunction; 4-17% progressed to end stage kidney disease and 4-11% died.[9111213] Poor outcomes in the modern era are likely related to older age and associated comorbidities like diabetes, alcoholism, malnutrition, malignancy etc.[8] By multivariate analysis, age and serum creatinine at biopsy correlated inversely with complete remission in adult patients with APIGN.[9]
The index case raises few important observations in APIGN to be addressed in the current era is the association of glomerular form of TMA interlinked or unrelated, normal complement levels, absence of preceding infection and role of steroids in APIGN.
Cases of GN with secondary malignant hypertension are described in crescentic GN, membranoproliferative GN and chronic GN.[14] However, APIGN causing secondary malignant hypertension is described in handful of cases.[34567] Some of the cases described in literature do not have histological counterpart of TMA. However, they fulfilled clinical and biochemical criteria. Majority of them were treated traditionally – fluid restriction and anti-hypertensive agents. An outcome in all patients was good, despite few required plasma exchange. Hypertension induced glomerular changes are typically wrinkling of basement membranes with shrinkage of tufts, followed by deposition of Periodic acid Schiff-negative hyaline material internal to bowmans capsule.[1415] Fibrinoid necrosis of tufts and TMA are rarely seen. Latter features are typically seen in patients developing malignant hypertension acutely. TMA is histologic finding from variety of causes requiring correlation with clinical findings. It is often secondary to shiga toxin, deficiency of alternate pathway of complement activation, auto immune diseases (anti-phospholipid antibody, systemic lupus erythematosus systemic sclerosis), hormonal imbalance, malignant hypertension, drugs (clopidegrol, ticlopidine), malignancy, radiation and in allografts (calcineurin inhibitor toxicity, antibody mediated rejection, recurrent glomerular disease).[16] The frequency of systemic TMA in cases with malignant hypertension has not been well described in the literature. Intrarenal TMA is relatively common and is a typical histological finding in malignant hypertension with renal impairment. If uncontrolled, the complication of TMA with renal dysfunction is devastating – cortical necrosis, partial or diffuse. Common factor between APIGN and TMA described so far is neuraminidase produced by streptococci. Neuraminidase injures vascular endothelium, initiating the cascade of thrombus formation.[346717] Our case is different from previous cases described in association with neuraminidase producing organisms, in that TMA is secondary to malignant hypertension substantiated by clinical findings of accelerated hypertension and hypertensive encephalopathy symptoms/radiologic findings.
Typical clinical description of APIGN in text book is “onset of acute nephritic syndrome 1-2 weeks after an antecedent streptococcal pharyngitis or 3 to 6 weeks after a streptococcal pyoderma.” However, study from Children's Hospital of Wisconsin shows that this is not the scenario always, addressing the factors for delay in diagnosing APIGN in their 5-year study period of 55 children. They have observed lack of preceding history of impetigo, sore throat, or a documented streptococcal infection in 26 out of 55 (56%) children studied.[18]
Complements are decreased in a majority of cases of APIGN and return to normal within about 6 weeks of onset of nephritis. Upto 24% of cases can have normal complement levels.[9] Analysing our case, we feel other infective agents producing immune-complexes possessing poor avidity to the complement activation as the reason for paucity of hypocomplementinemia. Absence of antistreptolysin-O titer in our case substantiates the non-streptococcal infection and hypothesis of normal level of complements.
Anti-inflammatory effect of steroid therapy might benefit severe forms of APIGN. Crucial judgement is to be based upon the level of renal insufficiency and presence or absence of crescents in administering steroid therapy. 33% of patients of APIGN In Columbia university Medical Center, 33% received steroid therapy (indications: Renal insufficiency with or without crescents) and found that no correlation existed between therapy and outcome.[9] Efficacy of steroid therapy for APIGN with crescentic transformation (>50% of glomeruli with crescents) is largely anecdotal.[19202122]
Conclusion
We present a case of presumably non-streptococcal APIGN with focal crescents and TMA secondary to malignant hypertension with end organ damage; highlighting the importance of timely urine analysis in managing GN.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Clinico-pathologic correlations in acute poststreptococcal glomerulonephritis. A correlation between renal functions, morphologic damage and clinical course of 46 children with acute poststreptococcal glomerulonephritis. Medicine (Baltimore). 1971;50:453-501.
- [Google Scholar]
- Acute poststreptococcal glomerulonephritis associated with thrombotic microangiopathy in an adult. Clin Nephrol. 2000;54:169-73.
- [Google Scholar]
- Simultaneous postinfectious glomerulonephritis and thrombotic microangiopathy: A renal biopsy study. Am J Kidney Dis. 1998;31:513-20.
- [Google Scholar]
- Hemolytic uremic syndrome complicating postinfectious glomerulonephritis in the adult. Am J Kidney Dis. 1995;25:336-9.
- [Google Scholar]
- Simultaneous occurrence of the haemolytic uraemic syndrome and acute post-infectious glomerulonephritis. Eur J Pediatr. 2001;160:173-6.
- [Google Scholar]
- An adult with acute poststreptococcal glomerulonephritis complicated by hemolytic uremic syndrome and nephrotic syndrome. Am J Kidney Dis. 2005;46:e59-63.
- [Google Scholar]
- Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013;83:792-803.
- [Google Scholar]
- Acute postinfectious glomerulonephritis in the modern era: Experience with 86 adults and review of the literature. Medicine (Baltimore). 2008;87:21-32.
- [Google Scholar]
- The current spectrum of infectious glomerulonephritis. Experience with 76 patients and review of the literature. Medicine (Baltimore). 1995;74:63-73.
- [Google Scholar]
- Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-32.
- [Google Scholar]
- Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol. 2011;22:187-95.
- [Google Scholar]
- The current state of poststreptococcal glomerulonephritis. J Am Soc Nephrol. 2008;19:1855-64.
- [Google Scholar]
- Malignant hypertension (a clinico-pathologic study of 43 cases) J Postgrad Med. 1987;33:49-54.
- [Google Scholar]
- Mesangiolysis: An important glomerular lesion in thrombotic microangiopathy. Mod Pathol. 1991;4:161-6.
- [Google Scholar]
- Microangiopathies and malignant vascular injury in the kidney. Curr Diagn Pathol. 2004;10:36-51.
- [Google Scholar]
- Acute renal failure, hemolytic anemia, and thrombocytopenia in poststreptococcal glomerulonephritis. South Med J. 1987;80:370-3.
- [Google Scholar]
- Delay in diagnosis in poststreptococcal glomerulonephritis. J Pediatr. 2008;153:560-4.
- [Google Scholar]
- Crescentic post-streptococcal glomerulonephritis with nephrotic syndrome in the adult: Is aggressive therapy warranted? Clin Nephrol. 2005;63:375-80.
- [Google Scholar]
- Crescentic glomerulonephritis in children: A review of 43 cases. Am J Nephrol. 1992;12:155-61.
- [Google Scholar]
- A rare case of postinfectious glomerulonephritis caused by pneumococcus in an adult patient. J Nephrol. 2007;20:99-102.
- [Google Scholar]
- Crescentic nephritis at Groote schuur hospital, South Africa: Not a benign disease. Clin Nephrol. 1994;42:22-9.
- [Google Scholar]







