Translate this page into:
Biopsy-proven renal disease in Ile-Ife, Nigeria: A histopathologic review
Address for correspondence: Dr. D. Sabageh, Department of Morbid Anatomy and Histopathology, Ladoke Akintola University of Technology Teaching Hospital, Ogbomoso, Nigeria. E-mail: dsabageh@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Although various patterns of renal diseases have been reported from different renal biopsy registries worldwide, data from Nigeria remain scanty. A 10-year retrospective review of renal biopsies was conducted in our tertiary health care facility. All cases were reclassified based on their light microscopic features after the application of standard histochemical stains. A total of 165 cases were reviewed with a male:female ratio of 1.8:1 and a mean age of 15.4 ± 12.0 years. About 69.7% of the cases were below the age of 16 years, while only 2.4% were older than 50 years. The most common indications for biopsy were nephrotic syndrome (72.1%) and acute renal failure of unknown etiology (11.5%). Overall, glomerulonephritis (80%) was the most common histologic category and occurred only in individuals younger than 50 years old. Minimal change disease (22.9%) and membranoproliferative glomerulonephritis (21.9%) were the most common varieties in children, while membranous glomerulonephritis (30.6%) and focal segmental glomerulosclerosis (27.8%) were the commonest among the adult population. The initial histologic diagnosis was revised in 18 cases while a diagnosis was arrived at in seven cases initially adjudged as inadequate for assessment. This study showed that renal biopsy was predominantly performed in children and adolescents. Although glomerulonephritis was the predominant disease, the predominant histologic patterns varied with the patient age. Despite the scarcity of advanced diagnostic tools in resource-poor environments, routine use of histochemical stains is helpful in the evaluation of renal biopsies.
Keywords
Histopathology
Nigeria
renal biopsy
Introduction
Ever since Ball performed the first closed needle biopsy in 1934, percutaneous renal biopsy has remained an essential part of the diagnostic work-up of patients with renal parenchymal diseases.[123] Tissues obtained through this procedure are not only pivotal in determining the cause of renal disease but are also very useful in predicting the prognosis and directing appropriate treatment.[2] Epidemiological studies using renal biopsies are vital for monitoring glomerular disease trends and establishing empirical principles that may aid the early detection and control of these diseases.[4]
Common indications for renal biopsies include nephrotic syndrome (NS), acute nephritic syndrome (ANS), non-nephrotic proteinuria (NNP), acute renal failure (ARF), especially where the disease is unresponsive to therapy and the etiology is not clinically apparent, and in patients presenting initially to the hospital in chronic renal failure (CRF) without an apparent clinical cause.[5]
The accurate diagnosis of renal disease typically requires the integration of clinical data, serological tests and complete pathological evaluation of the renal biopsy by light microscopy (LM), immunofluorescence (IF) and electron microscopy (EM).[678] Although this approach is routinely undertaken in developed countries, it remains a huge challenge in developing countries (including Nigeria) where the assessment of virtually all renal biopsies is now limited to LM using only routine hematoxylin and eosin (H and E) stain with the attendant diagnostic limitations.[26910] This notwithstanding, distinct histological changes characterize common renal disorders and can be readily visualized on LM, especially when performed with the aid of standard histochemical stains. Although renal diseases are an important cause of morbidity and mortality in Nigeria, data still remains scanty as most nephrologists do not perform renal biopsies.[11]
This study, therefore, aimed to evaluate renal biopsies using LM with the aid of standard histochemical stains, the ultimate goal being proper disease characterization and documentation of the pattern of renal and especially glomerular diseases as seen in our teaching hospital in Ile-Ife, Nigeria.
Materials and Methods
This is a retrospective study of all percutaneous renal biopsies received at the Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile-Ife, Nigeria, between January 2002 and December 2011. The hospital has about 576 beds and serves a population of over 1,333,603 with a slight female predominance (2006 census).
The surgical biopsy reports as well as the original request cards of the patients were scrutinized and all demographic and relevant clinical details were noted. Archival tissue blocks from the renal biopsy specimens were retrieved and fresh 2–4 µ sections cut, stained with H and E, periodic acid Schiff, reticulin, Jones silver methenamine, Masson's trichrome and Congo red (where indicated) stains and reviewed using a standard compound LM. Appropriate positive and negative tissue controls were used for each of the stains applied. Cases with inadequate tissue samples were excluded from the study.
The histological diagnoses were classified into four major categories, including primary or secondary glomerulonephritis, acute or chronic tubulointerstitial nephropathy, malignant neoplastic diseases and miscellaneous disorders, including but not limited to renal infarction, vascular diseases, normal histologies, graft rejection, and diabetic nephropathy.
The glomerulonephritides were classified according to the World Health Organization classification scheme.[12] Tubulointerstitial nephropathy comprised acute and chronic tubulointerstitial nephritis, acute or chronic pyelonephritis, acute tubular necrosis (ATN), renal cortical necrosis and reflux nephropathy.
The results were analyzed for differences in proportion using Chi-square by the Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, IL, USA). The frequencies of cases were expressed as percentages. The level of statistical significance was set at P ≤ 0.05.
Approval for the study was obtained from the local Ethics and Research Committee of the OAUTHC, Ile-Ife, Nigeria.
Results
A total of 264 renal biopsy specimens were received in our histopathology laboratory over the 10-year study period. Out of these, 32 were adjudged to be inadequate as they did not show any renal glomeruli while 28 were failed biopsies, which did not include any renal tissue. An additional 39 biopsies also had incomplete data and missing blocks and were also excluded from the study. Hence only 165 renal biopsies were reviewed and analyzed.
Of the 165 cases analyzed, there were 107 (64.8%) males and 58 (35.2%) females with a male:female ratio of 1.8: 1 and an overall mean age of 15.4 ± 12.0 years [Figure 1]. The age range was from 2 years to 60 years. About 115 cases (69.7%) were below the age of 16 years while there were only 46 cases (27.9%) between 16 years and 50 years old and just four cases (2.4%) older than 50 years [Figure 1].
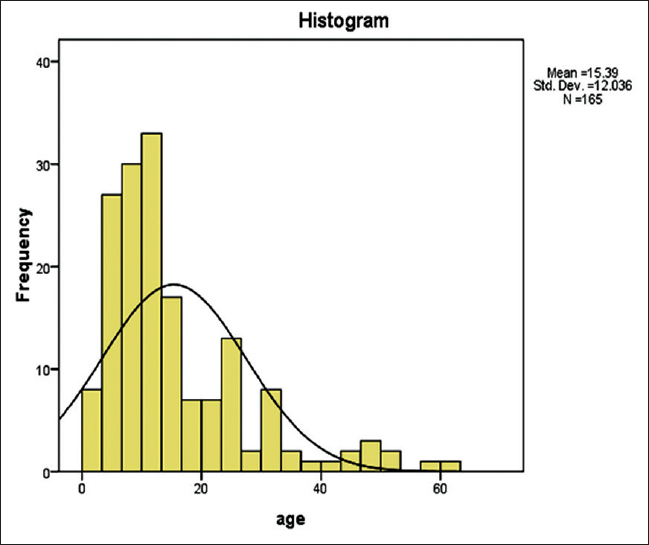
- Histogram showing age distribution of patients with renal disease
Table 1 shows that NS was the most common clinical indication for renal biopsy accounting for 119 (72.1%) cases while ARF accounted for 19 (11.5%) cases. On the other hand, patients presenting with ANS and CRF each accounted for eight (4.8%) cases. The least common indication was asymptomatic proteinuria which accounted for only two (1.2%) cases. NS was also the most common indication for renal biopsy in individuals younger than 16 years, accounting for 82 (71.3%) of such cases. It was also the most common indication in individuals aged between 17 years and 50 years. In contrast to this, asymptomatic urinary abnormality was the clinical indication in 50% of individuals older than 50 years old [Table 1]. All four cases suspected to be due to malignant infiltration were younger than 16 years old.

Table 2 shows that glomerulonephritis was the most common category of histological diagnosis, accounting for 132 (80.0%) of all biopsies as well as 83.5% and 78.3% of individuals in the 0–16 and the 17–50 year age groups, respectively. It was not seen in individuals over the age of 50 years. Moreover, tubulointerstitial nephritis and malignant neoplasia, respectively accounted for 13 (7.9%) and two (1.2%) cases and were only seen in individuals younger than 50 years old. In contrast to this, miscellaneous disorders were seen in all age groups and accounted for 18 (10.9%) cases. There were 13 cases of tubulointerstitial nephritis out of which eight (61.5%) were males and five (38.5%) were females [Table 2]. The mean age at diagnosis was 16.5 ± 10.6 years with an age range of 4–37 years. Acute and chronic interstitial nephritis accounted for four (30.8%) and nine (69.2%) cases, respectively. Only two renal biopsies showed malignant neoplastic infiltration both of which were diagnosed as Burkitt's lymphoma. Both cases were seen in children younger than 16 years old. Of the 18 miscellaneous cases, four (22.2%) were due to chronic transplant glomerulopathy, all of which occurred in patients over 40 years old. Others included six (33.3%) cases of ATN, four (22.2%) cases of acute cortical necrosis, and two (11.1%) cases each of diabetic nephropathy and end stage renal disease.

The most common glomerulonephritides encountered were membranoproliferative glomerulonephritis (MPGN) and minimal change disease (MCD) each accounting for 26 (19.7%) cases although focal segmental glomerulosclerosis (FSGS) and membranous glomerulonephritis (MGN) each accounted for 21 (15.9%) cases [Table 3]. The least common glomerulonephritides were focal proliferative glomerulonephritis (FPGN) and crescentic glomerulonephritis (CrGN) each accounting for two (1.5%) cases. These together with post-streptococcal glomerulonephritis (PSGN) were only seen below the age of 16 years. However, MCD and MPGN were the most common subtypes of glomerulonephritis in the 0–16 years age group accounting for 22.9% and 21.9% of cases, respectively while FPGN, CrGN and lupus nephritis were the least common histological subtypes within that age group accounting for 2.1% each of all cases in that age group. On the other hand, MGN and FSGS were the most frequent types of glomerulonephritis in the 17–50 years age group accounting for 30.6% and 27.8% of cases respectively while PSGN, FPGN and CrGN were not seen in this age group. No glomerular lesions were seen in cases above the age of 50 years.

All histologic types of glomerulonephritis were more prevalent in males except for FPGN and CrGN which both showed equal sex distribution and lupus nephritis which was only seen in females [Table 3]. In males, MPGN was the most common histologic type accounting for 22.1% of cases while FSGS accounted for 19.8% of male cases. In contrast to this, MCD was the most common type in females accounting for 23.9% of female cases although MGN also accounted for 17.4% of female cases. The least common subtypes in females were FPGN (1.5%) and CrGN (1.5%).
There were 18 cases in which the original diagnosis was revised after the application of a standard panel of histochemical stains [Table 4]. The ages of the patients ranged from 4 years to 28 years. There were 14 males and four females. In about 14 (77.8%) cases, the original diagnosis was MCD. The final diagnoses were MGN (33.3%), FSGS (22.2%), MPGN (22.2%), mesangioproliferative glomerulonephritis (MesPGN) (11.1%), PSGN (5.6%) and MCD (5.6%). Table 4 also shows the seven cases in which there was no previous diagnosis before the application of histochemical stains. Five of these were initially adjudged to be inadequate. The ages ranged from 5 years to 22 years. There were five males and two females. The final diagnoses included two cases each of MCD and MGN as well as one case each of MPGN, MesPGN and FSGS.

Figure 2 shows the distribution of the major categories of biopsy-proven renal diseases over the study period. Glomerulonephritis consistently represented the dominant histological diagnosis throughout the study period with three peaks in 2003, 2008 and 2011. The number of biopsies performed ranged from 7 per year in 2005 to 27 per year in 2008.

- Distribution of the various categories of histological diagnosis over the course of the study period
Discussion
It is well established that the pattern of renal diseases varies according to the geographical location, race, age, sex, socioeconomic conditions and clinical indications for the renal biopsies. Nevertheless, the overall male preponderance noted in this survey may suggest that renal parenchymal diseases occur more frequently in our male population. To buttress this fact, of all the glomerular lesions encountered, only MesPGN showed a higher number of cases in females. This is similar to records obtained from various parts of the world, including the Italian registry of renal biopsies, the United Kingdom Medical Research Council's glomerulonephritis registry and the United States Renal Data System annual report, which all show that males were more represented in biopsy-proven renal diseases.[13] A few other studies have, however, reported a female preponderance in their series while yet, others have shown an equal sex distribution.[141516] These variations in the overall sex distribution are also reflected among the various categories of renal diseases in the different geographical locations earlier mentioned. It is interesting to note, however, that many of the series that show an overall female preponderance actually demonstrated a significantly high proportion of females with lupus nephritis.[1516]
Our study also showed a much lower mean age than what has been reported in various studies from different parts of the world.[24131516] This is not surprising since about 69.7% of the patients in our study were younger than 16 years old. While this may erroneously suggest that renal diseases are more prevalent in our childhood population, it may actually be due to the fact that fewer biopsies were performed on adults in our hospital during the study period. This may not be unconnected with the fact that majority of our adult patients present late to hospital at a time when renal biopsies are no longer indicated for their initial diagnostic workup and management. In fact, a previous study done in the same center showed that 89.4% of all the patients who died from CRF over a period of 5 years were older than 20 years old.[17] During this time, diagnosis of CRF was made by clinical, radiological and biochemical parameters without recourse to renal biopsy.[17] Interestingly, our hospital has a better developed adult renal unit with a dedicated adult renal ward, dialysis and transplant programs unlike the pediatric section.
Just like various reports from different parts of the world, NS was the most common clinical indication for renal biopsy in our hospital.[18192021] This, however, contrasts with reports from Hong Kong where persistent NNP was reported to be the major indication for renal biopsy.[1] Although this may reflect differences in the renal biopsy practices between the two centers, it may actually be due to differences in the etiopathogenesis of renal disorders between these regions. Indeed, it has been observed that exposure to the same primary or secondary etiological agent may result in various patterns of glomerular injury in different individuals and that this may be related to their ethnic origin and specific human leukocyte antigen haplotypes.[11]
Glomerulopathies constituted the largest group in our series accounting for about 80% of all cases of biopsy-proven renal disease. This is similar to various reports from different parts of the world and seems to underscore the importance of glomerular lesions in the pathogenesis of renal disease.[2223] Interestingly, while more than 80% of these glomerulopathies were diagnosed in children younger than 16 years old, none was recorded in any patient over the age of 50 years. It is possible that glomerular lesions in our environment occur more commonly in children but rare after the age of 50 years. On the contrary, this may just be due to the fact that relatively fewer biopsies are performed on adults in our hospital as previously explained.
Although it is well recognized that the histopathological patterns of glomerular diseases documented by various authors have usually been at variance with one another, different patterns of glomerulonephritis are more commonly associated with particular age groups.[24] In fact, earlier studies in some parts of Nigeria have reported higher incidence rates for MPGN but much lower values for MCD in children.[222526] Our study, however, showed that MCD and MPGN were the most prevalent forms of glomerulonephritis seen in the pediatric age group even though a significantly high proportion of our pediatric cases were also due respectively to MesPGN, MGN, FSGS and PSGN. These fairly common histologic varieties have been found to also contribute significantly to renal diseases in Senegal, Jordan and Brazil (FSGS) as well as Hong Kong, USA and Australia (IgA nephropathy).[11318272829] The increasing role played by FSGS and MGN in the pathogenesis of glomerular disease in children, as corroborated by our study, may suggest changes in the etiopathogenesis of glomerular lesions among our patients possibly related to rising levels of environmental pollution and childhood obesity.[24] These forms of glomerulonephritis are, however, known to constitute the bulk of steroid resistant cases in children.[24] In sharp contrast to this, however, our study showed that MGN and FSGS constituted the major histopathological types of glomerulonephritis in the adult population while MCD, MesPGN and MPGN were relatively few. The high frequency of MGN and FSGS and the low frequency of MCD in the Nigerian adult population are well recognized although some authors have reported contrasting findings.[11] While the differences in the predominant histologic patterns of glomerulonephritis between the pediatric and adult populations may suggest differences in their etiopathogenesis, they may also explain some of the relative differences observed in their overall clinical outcomes and responses to therapy.[2430]
Tubulointerstitial nephropathy constituted only 7.9% of all renal diseases in this study. This relatively low frequency suggests a low prevalence of such lesions in our population although it may have resulted from a low rate of biopsy for such cases especially patients with CRF, a condition with which tubulointerstitial nephritis is frequently associated. In fact the absence of drug-induced interstitial nephritis in this study, a very common cause of interstitial nephritis in our environment, is very instructive.[13] It, therefore, becomes imperative that a high index of suspicion is developed for such cases so that appropriate investigations including renal biopsies may be performed as this will form the basis for the institution of the appropriate therapy.
The fact that, in 15.2% of the biopsies in this study, the initial diagnosis, which was based solely on routine H and E, was revised after the application of histochemical stains, underscores the important role these special stains play in the routine evaluation of renal biopsies. In fact, findings from this study seem to suggest that a diagnosis of MCD should not be entertained without the application of these special stains as MCD was the initial diagnosis in 14 of the 18 cases so revised. Thus, despite the non-availability of IF techniques and EM in many resource-poor countries including Nigeria, histochemical stains should be routinely employed in the evaluation of renal biopsies as this would go a long way in improving the diagnostic yield obtainable from the biopsies. Nevertheless, IF and EM still remain the ultimate diagnostic techniques in the evaluation of renal biopsies.
According to this study, there was no variation in the overall pattern of biopsy-proven renal diseases as glomerulonephritis remained the predominant histologic diagnosis for each year of the study period. This seems to suggest that the putative etiological factors responsive for the major groups of renal disorders in our environment have remained relatively constant over the years. It, therefore, becomes imperative to identify these factors in the hope that this would help in instituting appropriate preventive measures.
Conclusion
This study showed that the majority of renal biopsies received at our center during the period under review were from male children below the age of 16 years. NS was the most common indication for the renal biopsies performed, while glomerular diseases accounted for the majority of the renal lesions encountered. MCD and MPGN were the most common glomerulonephritides in the pediatric age group while MGN and FSGS were the most common types among the adult population. Nevertheless, MesPGN, FSGS, MGN and PSGN appear to be playing increasingly more important roles in the pediatric age group. Glomerular lesions were not seen after the age of 50 years while lupus nephritis was also relatively uncommon.
It is our hope that the information obtained from this study will aid our understanding of renal disease among the Nigerian population. It is also hoped that these findings may form a foundation for further research into different aspects of renal diseases in our environment.
This study is limited by the inability of the authors to perform IF and/or EM in order to fully characterize the glomerulopathies. Interestingly, it is possible that majority of the cases in our series diagnosed as mesangial proliferative glomerulonephritis are actually due to IgA nephropathy, a disease that requires IF and EM for definitive diagnosis.[31] Furthermore, the findings from this study may not necessarily be applicable to the general population as the study was carried out in a single tertiary health institution, which may have led to a selection bias.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Clinical and pathological characteristics of patients with glomerular diseases at a university teaching hospital: 5-year prospective review. Hong Kong Med J. 1999;5:240-244.
- [Google Scholar]
- Histological pattern of glomerulopathies at Khyber teaching hospital, Peshawar. Pak J Med Res. 2004;43:117-20.
- [Google Scholar]
- Biopsies and the Completion of certain Surgical Procedures. Can Med Assoc J. 1923;13:820-3.
- [Google Scholar]
- Kidney biopsy in west of Iran: Complications and histopathological findings. Indian J Nephrol. 2009;19:68-70.
- [Google Scholar]
- Pattern of renal diseases observed in native renal biopsies in adults in a single centre in Pakistan. Nephrology (Carlton). 2011;16:87-92.
- [Google Scholar]
- Laboratory investigation of renal biopsy specimen. J Nephrol Urol Transplant. 1998;1:19-26.
- [Google Scholar]
- Electron microscopy and immunocytochemistry in the assessment of renal biopsy specimens: Actual and optimal practice. J Clin Pathol. 1996;49:233-7.
- [Google Scholar]
- Application of immunoperoxidase (PAP) technique for demonstration of deposited immunoglobulins in renal biopsies. J Pak Med Assoc. 1988;38:66-9.
- [Google Scholar]
- The diagnosis of glomerular diseases: Acute glomerulonephritis and the nephrotic syndrome. Arch Intern Med. 2001;161:25-34.
- [Google Scholar]
- Clinicopathologic study of adult nephrotic syndrome in Ilorin Nigeria. Niger Med Pract. 2003;43:28-32.
- [Google Scholar]
- Renal Disease: Classification and Atlas of Glomerular Diseases. (2nd ed). New York: Igaku-Shoin; 1995.
- [Google Scholar]
- Survey of the Italian registry of renal biopsies. Frequency of the renal diseases for 7 consecutive years. The Italian Group of Renal Immunopathology. Nephrol Dial Transplant. 1997;12:418-26.
- [Google Scholar]
- An overview on frequency of renal biopsy diagnosis in Brazil: Clinical and pathological patterns based on 9,617 native kidney biopsies. Nephrol Dial Transplant. 2010;25:490-6.
- [Google Scholar]
- Patterns of renal disease in Cape Town South Africa: A 10-year review of a single-centre renal biopsy database. Nephrol Dial Transplant. 2011;26:1853-61.
- [Google Scholar]
- The histopathological profile of kidney diseases in a single centre in Egypt: An overview of 14 years of experience. J Clin Diagn Res. 2011;5:295-300.
- [Google Scholar]
- The pathological basis of chronic renal failure in Nigerians. An autopsy study. Trop Geogr Med. 1992;44:42-6.
- [Google Scholar]
- Histopathological profiles of nephropathies in senegal. Saudi J Kidney Dis Transpl. 2003;14:212-4.
- [Google Scholar]
- Spsnish Registry of Glomerulonephritis. Frequency of renal pathology in Spain 1994-1999. Nephrol Dial Transplant. 2002;17:1594-602.
- [Google Scholar]
- Changing prevalence of glomerular diseases in Korean adults: A review of 20 years of experience. Nephrol Dial Transplant. 2009;24:2406-10.
- [Google Scholar]
- Spectrum of glomerulonephritis in Egypt. Saudi J Kidney Dis Transpl. 2000;11:421-9.
- [Google Scholar]
- Clinicopathological features of childhood nephrotic syndrome in northern Nigeria. Q J Med. 1990;75:563-76.
- [Google Scholar]
- Epidemiology of renal disease in Romania: A 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant. 2006;21:419-24.
- [Google Scholar]
- Nephrotic syndrome among children in Kano: A clinicopathological study. Niger J Clin Pract. 2014;17:370-4.
- [Google Scholar]
- The predominance of membranoproliferative glomerulonephritis in childhood nephrotic syndrome in Ibadan, Nigeria. West Afr J Med. 1999;18:203-6.
- [Google Scholar]
- Spectrum of glomerulonephritis in adult Jordanians at Jordan university hospital. Saudi J Kidney Dis Transpl. 2008;19:997-1000.
- [Google Scholar]
- Changing incidence of glomerular disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol. 2006;1:483-7.
- [Google Scholar]
- Renal biopsy registry from a single centre in Serbia: 20 years of experience. Nephrol Dial Transplant. 2009;24:877-85.
- [Google Scholar]
- IgA nephropathy (IgAN) presenting with the nephrotic syndrome. Trop Geogr Med. 1992;44:365-8.
- [Google Scholar]







