Translate this page into:
C3 Dominant Collapsing Focal Segmental Glomerulosclerosis – A Report of Two Rare Cases
Corresponding Author: Dr. Mythri Shankar, 926, Anugraha, Ground Floor, 22nd Cross, 5th Main Sector 7, HSR Layout, Bengaluru, Karnataka, India. E-mail: mythri.nish@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shankar M, Gurusiddiah SC, Vinay KS, Aralapuram K, Siddalingappa R, Satheesh G. C3 Dominant Collapsing Focal Segmental Glomerulosclerosis – A Report of Two Rare Cases. Indian J Nephrol 2024;34:70-3. doi: 10.4103/ijn.ijn_250_22
Abstract
Collapsing focal segmental glomerulosclerosis (FSGS) a heterogeneous group of disorders, rather than a single disease entity. Kidney biopsy shows segmental or globally collapsed, sclerotic glomerular capillaries. There is also hypertrophy and hyperplasia of overlying glomerular epithelial cells. Immuno-fluorescence is negative or has non-specific deposits of immunoglobulins and C3. We present two cases of C3 dominant collapsing FSGS. Both the cases were non-responsive to therapy and had a poor outcome. This calls for research to study the role of the complement pathway in the pathogenesis of FSGS.
Keywords
C3 glomerulopathy
collapsing FSGS
Introduction
Collapsing focal segmental glomerulosclerosis (FSGS) is most commonly seen in patients with human immuno-deficiency virus (HIV) infection. It is called HIV-associated nephropathy (HIV-AN). Collapsing FSGS has been increasingly recognized in non-HIV patients as well. It is now considered a heterogeneous group of disorders rather than a single disease entity.1 Kidney biopsy shows segmental or globally collapsed, sclerotic glomerular capillaries. There is hypertrophy and hyperplasia of overlying glomerular epithelial cells. Immuno-fluorescence is usually negative or has non-specific deposits of immunoglobulins and C3. A retrospective study by Mirioglu. S et al.2 showed that co-deposition of IgM and C3 in FSGS is associated with poor prognosis. One case of C3 dominant collapsing FSGS is presented in the glomcon case discussion.3 However, to the best of our knowledge, there are no case reports of isolated dominant C3 mesangial deposits in collapsing FSGS. We present two cases of C3 dominant collapsing FSGS.
Case Report
Case 1
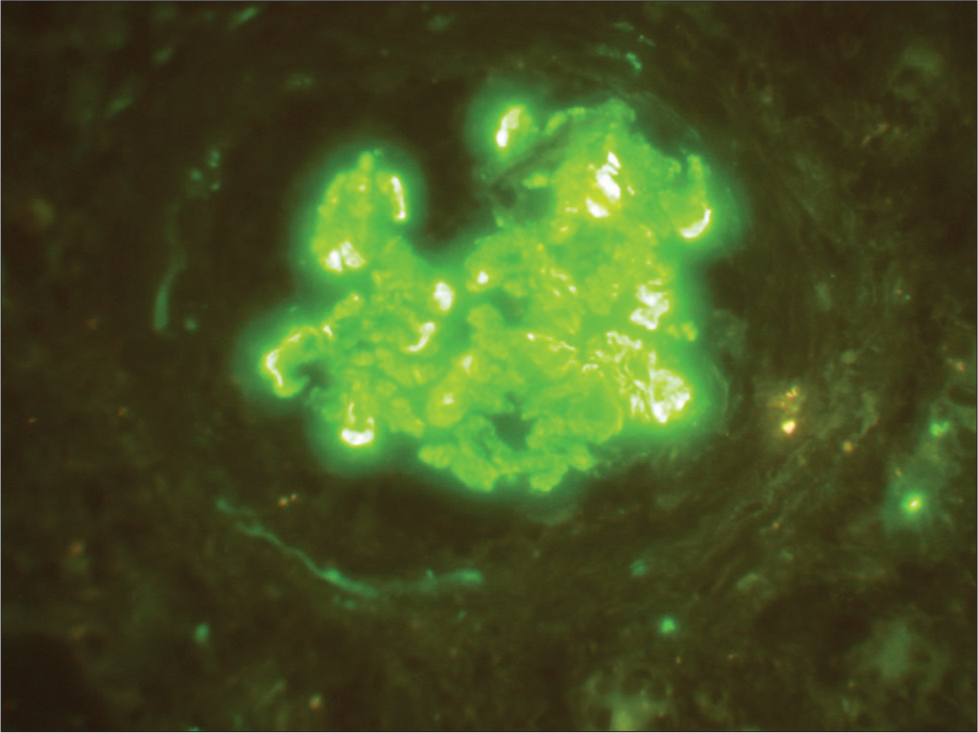
A 14-year-old female (case 1) presented with facial puffiness, swelling of bilateral feet, and dyspnoea for 2 months. The symptoms were acute in onset and progressed over a period of 2 months. These symptoms were associated with frothy urine. There was no history of hematuria. She had no significant family history. She had no history suggestive of any significant infection in the past. There was no history of weight loss or use of any drugs/medications. On evaluation, she was found to be hypertensive, with pedal edema and bilateral reduced air entry in the basal lung fields, suggestive of volume overload. Clinical and laboratory findings [Table 1] suggested nephrotic syndrome with acute kidney injury (AKI). Light microscopy [Images 1 and 2] on kidney biopsy showed collapsing FSGS. There was marked hyperplasia of podocytes over the collapsed areas of the tuft. Protein resorption droplets were found in the proximal convoluted tubules. Immuno-fluorescence (IF) [Image 3] revealed predominant C3 deposits (3+) in the mesangium, with negative IgG, IgM, IgA, and C1q. She was initiated on optimal RAS (renin-angiotensin system) inhibition, blood pressure control, and treatment of dyslipidemia. After ruling out active infection, she was started on steroids. Unfortunately, she progressed to end-stage kidney disease over a period of 2 years.
| Investigations | Case 1 | Case 2 |
|---|---|---|
| Age (years) | 14 | 31 |
| Gender | female | female |
| Urine routine | Protein - 3+, | Protein - 3+ |
| (Protein, RBC - per high per field) | RBC - 5-6 | RBC- nil |
| Dysmorphic RBCs | Absent | Absent |
| 24-hour urine protein (in grams) | 10.5 | 8 |
| Hemoglobin (g%) | 8.4 | 11.6 |
| Total count (cells/mm3) | 9200 | 5530 |
| Platelet count (cells/mm3) | 4,95,000 | 2,58,000 |
| S.Creatinine (mg/dl) | 2.6 | 3.76 |
| B.Urea (mg/dl) | 66 | 57 |
| S.Sodium (mmol/L) | 135 | 141 |
| S.Potassium (mEq/L) | 3.7 | 4.8 |
| S.Calcium (corrected for S.albumin) (mg/dl) | 8.4 | 8.1 |
| S.Phosphorous (mg/dl) | 4.55 | 3.7 |
| S.Albumin (g/dl) | 2 | 3.5 |
| Total bilirubin (mg/dl) | 0.13 | 0.3 |
| Direct bilirubin (mg/dl) | 0.1 | 0.2 |
| SGOT | 13 | 19 |
| SGPT | 6 | 28 |
| Complement C3 | 75 | 61 |
| (normal range 80 IU/L to 180 IU/L) | ||
| Complement C4 | normal | normal |
| 2D Echo | Within normal limits | Hypertensive heart disease, EF - 61% PASP- 32 mmhg |
| Peripheral smear | Normocytic normochromic anemia | Normocytic normochromic anemia |
| Random blood glucose (mg/dl) | 110 | 110 |
| HIV/HBSAG/HCV | Negative | Negative |
| ANA | Negative | Negative |
| S.Ferritin (ng/ml) | 158 | 234 |
| Anti-Factor H Antibody levels | Within normal limits | Within normal limits |
| Serum protein electrophoresis | Negative for “M” band | Negative for “M” band |
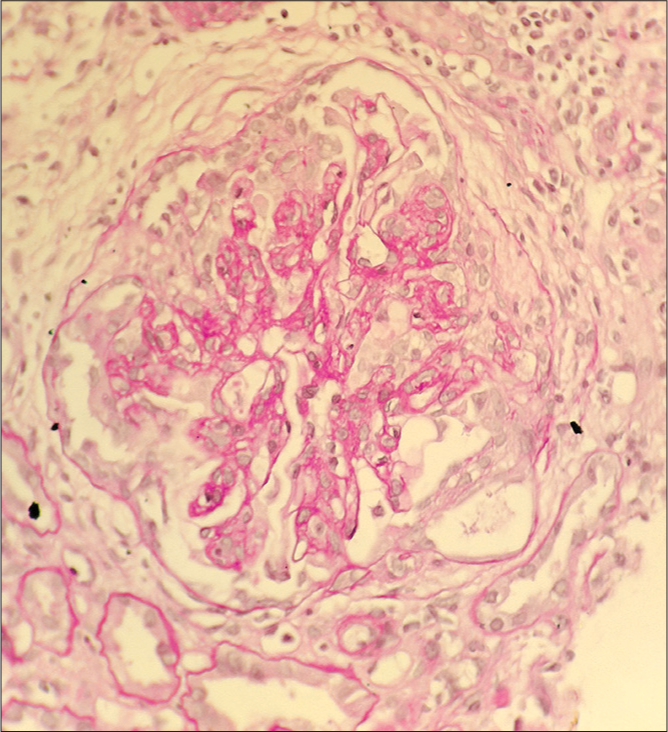
- Case 1: Light microscopy (PAS stain) – Collapsing FSGS.
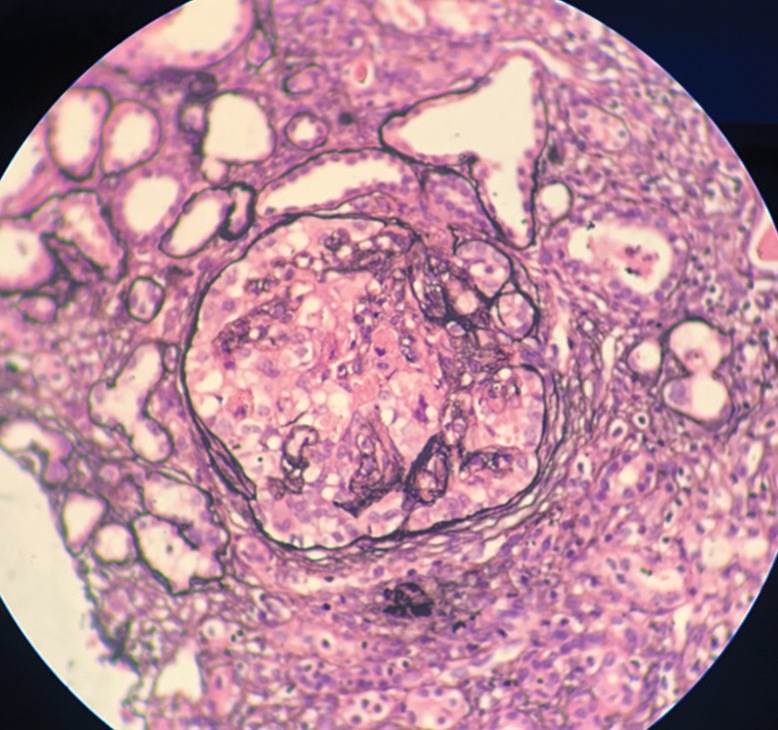
- Case 1: Light microscopy (silver stain): Collapsing FSGS.

- Case 1: Immunofluorescence: Predominant C3 mesangial deposits.
Case 2
A 37-year-old lady presented with symptoms of volume overload for 10 days. On physical examination, she was found to be hypertensive with pedal edema and crepitations in bilateral lung fields suggestive of volume overload. Clinical and laboratory findings [Table 1] suggested nephrotic syndrome with AKI. Light microscopy features showed collapsing FSGS with predominant C3 mesangial deposits on IF. (Images 4-6). She was initiated on optimal RAS (renin-angiotensin system) inhibition, blood pressure control, and treatment of dyslipidemia. She was also initiated on immuno-suppression with steroids, but unfortunately, she progressed to end-stage kidney disease in one year’s time.
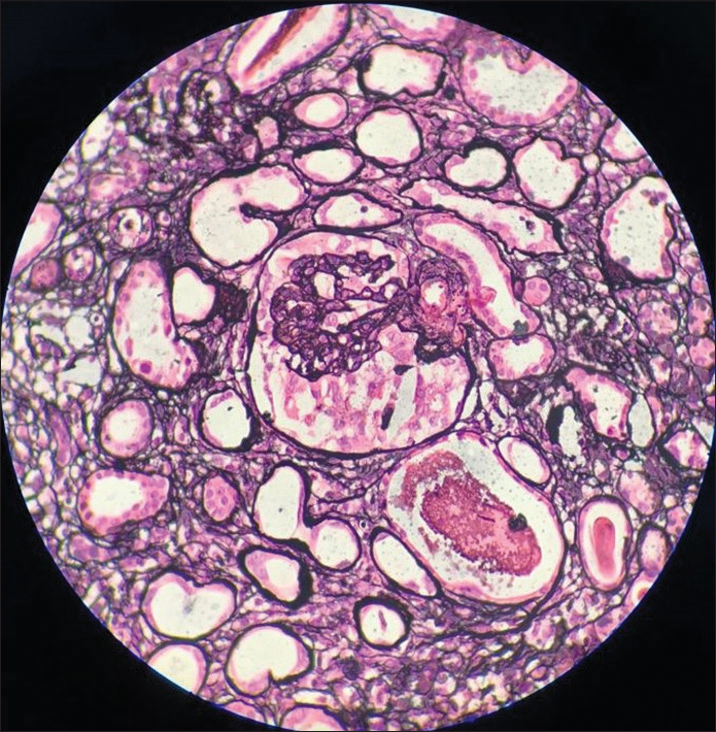
- Case 2: Light microscopy (silver stain): Collapsing FSGS.
- Case 2: Immunofluorescence: Collapsing FSGS.

- Electron microscopy of FSGS.
Discussion
In this case report, we describe the clinical features, kidney biopsy findings, poor response to therapy, and progression to the end-stage kidney disease in C3 dominant collapsing FSGS. We found that C3 dominant collapsing FSGS was non-responsive to steroids, and was associated with a poor prognosis.
Collapsing FSGS is characterized by segmental or global collapse of glomerular capillaries, with hypertrophy and hyperplasia of podocytes. Most of the cases are idiopathic, followed by infections, predominantly HIV. Other infections include parvovirus B19, malaria, leptospirosis, severe acute respiratory syndrome coronavirus 2, and drugs such as pamidronate.4 In the above cases, secondary causes for collapsing FSGS were ruled out. Patients with collapsing FSGS usually present with nephrotic syndrome and AKI, like the two cases discussed here. Two highly suggestive factors of collapsing FSGS are the clinical presentation of nephrotic range proteinuria with AKI and microcystic tubular changes, both of which were seen in these cases.4
C3 glomerulopathy is defined as predominant C3 deposits of order 2 or more than the other immunoglobulins or isolated dominant (2+ or more) C3 deposits. The patients had predominant C3 deposits in collapsing FSGS. This is suggestive of alternate complement pathway activation.5 Activation of the alternate pathways is because of genetic causes or immune complex-mediated. Serum C3 levels were low in both cases. Anti-factor H antibody levels were negative. Because of financial constraints, other genetic tests for alternate complement pathway activation could not be performed. Monoclonal gammopathy was ruled out by serum protein electrophoresis, and also, there were no restriction with Kappa/Lambda chains on IF microscopy.
To our knowledge, these two cases are the first to be reported with collapsing FSGS and dominant C3 deposits on IF. This raises the question of probable alternate complement pathway-mediated pathogenesis of collapsing FSGS. The proposed hypothesis is because of the pro-inflammatory cytokine (interferon 1) released during immune activation, which up-regulates the expression of the APOL1 mutant gene causing direct injury to glomerular epithelial cells.6 APOL1 and mRNA are normally expressed in the glomerular epithelial cells.7 Susceptibility genes for collapsing FSGS include APOL1 (most common) and genes involved in mitochondrial functions such as co-enzyme Q10 and PDSS2 gene.8
Complement activation has been demonstrated in animal models of adriamycin-induced FSGS, which was associated with progression to glomerulosclerosis.9 IgM and C3 co-deposition has been demonstrated in the sclerotic segments of FSGS and their association with poor response to treatment and poor prognosis.10 IgM is a pentamer immunoglobulin that strongly activates the complement.11 Systemic activation of the complement was recently demonstrated in humans with primary FSGS. Damage to the kidney was caused by membrane attack complex (MAC) and C5a. Urinary levels of Bb were associated with worse kidney outcomes. However, this study did not include patients with collapsing FSGS.12 This highlights the role of the complement in primary FSGS and the possible use of complement therapies in the management of such cases.
From the nephropathology point of view, the presence of bright C3 deposits in the absence of immunoglobulin deposits should raise the possibility of a defect in the alternate complement pathway. Low C3 and normal C4 also direct toward alternate complement pathway activation. Such cases merit extensive investigation to look for defects in the alternate complement pathway such as genetic mutations, Factor H antibodies, C3 nephritic factor, and so on. As it is a relatively recently diagnosed entity, the treatment recommendations are limited.13
C3 dominant collapsing FSGS is a risk factor for non-responsiveness to treatment, worsening kidney functions, and poor prognosis. Further research is required to evaluate the role of the alternate complement pathway in the pathogenesis of collapsing FSGS, and thereby targeted anti-complement therapy can be tried which may be helpful in these selective cases.
Acknowledgement
We acknowledge the Director, Faculty and residents of Department of Nephrology, Institute of Nephrourology, Bengaluru for their administrive and technical help during the workup of these cases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Kidney Blood Press Res. 2019;44:961-72.
- [CrossRef] [PubMed] [Google Scholar]
- C3 glomerulopathy-understanding a rare complement-driven renal disease. Nat Rev Nephrol. 2019;15:129-43.
- [CrossRef] [PubMed] [Google Scholar]
- Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int. 2015;87:332-42.
- [CrossRef] [PubMed] [Google Scholar]
- Localization of APOL1 protein and mRNA in the human kidney: Nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol. 2015;26:339-48.
- [CrossRef] [PubMed] [Google Scholar]
- COQ2 nephropathy: A newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773-80.
- [CrossRef] [PubMed] [Google Scholar]
- Complement activation contributes to both glomerular and tubulointerstitial damage in adriamycin nephropathy in mice. J Immunol. 2006;177:4094-102.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of IgM and C3 glomerular deposition in primary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2016;11:1582-9.
- [CrossRef] [PubMed] [Google Scholar]
- How antibodies use complement to regulate antibody responses. Mol Immunol. 2014;61:79-88.
- [CrossRef] [PubMed] [Google Scholar]
- Complement activation profile of patients with primary focal segmental glomerulosclerosis. PLoS One. 2020;15:e0234934. doi: 10.1371/journal.pone.0234934
- [CrossRef] [PubMed] [Google Scholar]
- C3 glomerulonephritis: Clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012;82:465-73.
- [CrossRef] [PubMed] [Google Scholar]







