Translate this page into:
C3 Glomerulonephritis Associated with Monoclonal Gammopathy
Corresponding author: Ana Daniela Piedade, Department of Nephrology, Setubal Hospital Center, Setúbal, Portugal. E-mail: anapereira.piedade@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Piedade AD, Domingues PA, Inácio AS, Mendes BB, Parreira LF. C3 Glomerulonephritis Associated with Monoclonal Gammopathy. Indian J Nephrol. 2024;34:660-2. doi: 10.25259/ijn_543_23
Abstract
C3 glomerulonephritis (C3GN) is rare. It is a proliferative glomerulonephritis resulting from glomerular deposition of complement factors due to dysregulation of the alternative pathway of complement. The association between monoclonal protein production and development of C3GN was described. We report a 74-year-old man with rapid worsening of kidney function and dialysis needed due to C3GN. In this case, serum protein electrophoresis showed no monoclonal spike but serum immunoelectrophoresis showed a lambda light chain monoclonal gammopathy. Once the diagnosis was made, the patient was treated with immunosuppression with complete kidney recovery. This case shows the importance of accurate and prompt diagnosis and appropriate treatment.
Keywords
Glomerulonephritis
C3 glomerulonephritis
Monoclonal gammopathy of renal significance
Membranoproliferative glomerulonephritis
Monoclonal gammopathy
Introduction
C3 glomerulopathies (C3G) are a rare group of kidney diseases affecting children or young adults with an incidence of one to three cases per million/year. This term was adopted in 2013 by expert’s consensus with the recognition of two subgroups, C3 glomerulonephritis (C3GN) and dense deposit disease (DDD), according to the distribution of electron-dense deposits on electron microscopy.1-3
C3GN is proliferative glomerulonephritis resulting from glomerular deposition of complement factors due to dysregulation of the alternative pathway (AP) of complement, which may occur as a result of mutations or functional inhibition of complement regulating proteins, such as mutations in complement factor H (CFH), complement factor B (CFB), complement factor I (CFI), membrane cofactor protein (MCP), complement component 3 (C3), or presence of nephritic factor C3 or other antibodies such as anti-factor H.2
C3GN presentation ranges from hematuria, proteinuria (with or without nephrotic syndrome), hypertension, and nephritic syndrome to rapidly progressive glomerulonephritis. Serum C3 levels are low in most patients.1,2
Monoclonal gammopathy of renal significance (MGRS) is a hematologic disorder caused by a monoclonal protein (M-protein) secreted by a small plasma cell clone or other B-cell clones in patients who do not meet the diagnostic criteria for multiple myeloma or other B-cell malignancies.4 The morbidity of MGRS is high when kidney dysfunction is present. Early diagnosis is crucial to stop the clonal production of immunoglobulins.4,5
The association between monoclonal protein production and development of C3GN has rarely been described.1,2
Case Report
A 74-year-old man with past history of diabetes mellitus, renal lithiasis, and psoriasis was admitted due to general malaise. Laboratory results showed rapid worsening of kindey function, with serum creatinine (SCr) 6.7 mg/dL (baseline 0.97 mg/dL), proteinuria 8.7 g/24 h, microscopic hematuria, and anemia. He was hypertensive, without peripheral edema. On admission, he had nephritic syndrome and rapidly progressive renal failure. During hospitalization, with the progressive worsening of renal dysfunction, hemodialysis was started.
There was a reduction in complement C3 (75 mg/dL), with normal C4 (27 mg/dL) and CH50. Serum levels of immunoglobulins were within normal ranges. Serum protein electrophoresis (SPE) showed no monoclonal spike, but serum immunoelectrophoresis (SIEP) showed a lambda light chain monoclonal gammopathy. Quantification of the free light chains showed kappa 15.8 mg/L, lambda 6 mg/L, and a free kappa/free lambda ratio of 2.6. Infectious, autoimmune, and complement-associated diseases were ruled out [Table 1]. Renal ultrasound did not show any relevant alterations. Bone marrow aspirate showed 1.5% plasma cells with a normal phenotype.
| Investigation | |
|---|---|
| Autoimmunity (antinuclear antibody, extractable nuclear antigen antibodies, antineutrophil cytoplasmic antibody, anti-glomerular basement membrane antibody, anti-dsDNA) | Negative |
| Anti-streptolysin O test | Negative |
| Human immunodeficiency virus, hepatitis B virus, and hepatitis C virus serologic tests | Negative |
| Molecular study of complement genes (CFH, CFB, CFI, MCP, C3, THBD, CFHR5, CFHR1, CFHR3, CFHR4, DGKE) | Negative |
| Nephritic factor C3 | Negative |
| Anti-factor H antibody | Negative |
C3: complement component 3, CFB: complement factor B, CFH: complement factor H, CFHR1: complement factor H related 1, CFHR3: complement factor H related 3, CFHR4: complement factor H related 4, CFHR5: complement factor H related 5, CFI: complement factor I, DGKE: diacylglycerol kinase epsilon, MCP: membrane cofactor protein, THBD: thrombomodulin
A kidney biopsy (KB) was performed. On light microscopy, all glomeruli showed global endocapillary proliferation with lobulation and double contours [Figure 1]. A circumferential cell crescent was observed in a glomerulus [Figure 2]. Subendothelial deposits were observed. 50% of the cortex was fibrous. Presence of diffuse fibroedema.
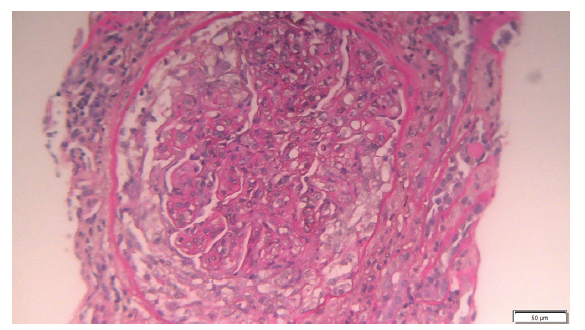
- Circumferential cell crescent in a glomerulus with global endocapillary proliferation, lobulation, and double contours (light microscopy 20x).
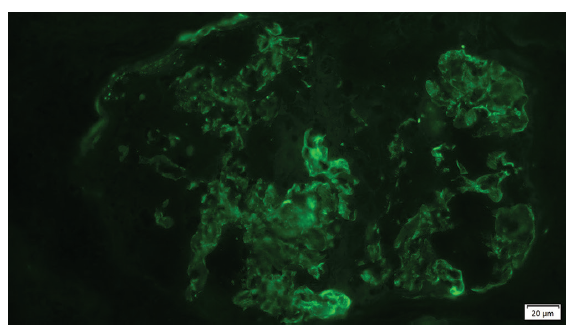
- Granular deposits of C3 in the mesangium and capillary (immunofluorescence).
Intense mononuclear inflammatory infiltrate was observed, as well as acute tubular necrosis and intratubular erythrocytes; Immunofluorescence showed granular deposits of C3 in the mesangium and capillary wall (3+) [Figure 2]. However, it was negative for IgA, IgG, IgM, fibrinogen. It did not show restriction of kappa and lambda light chains. The same was observed in the capillary walls; KB showing a C3 glomerulopathy with membranoproliferative pattern.
A genetic study was carried out to search for mutations in the components of the alternating complement pathway, which did not reveal alterations [Table 1].
Therapy was started with corticosteroids (methylprednisolone and prednisolone 1 mg/kg/day) and cyclophosphamide pulses, which were switched at the fourth month to mycophenolate mofetil. After 5 months: SIEP was negative twice and the patient’s kidney function recovered, and therefore, dialysis was stopped at the end of the first month of therapy. After 12 months of follow-up, SCr was 1.6 mg/dL with 0.34 g/g of urine protein/creatine ratio.
Discussion
C3GN is a rare type of glomerulonephritis resulting from dysregulation of AP of the complement. The mechanism by which monoclonal Ig results in activation of AP of the complement is not entirely known, but likely involves activation of AP by the aberrant protein. The hallmark of the disease is the presence of C3 on immunofluorescence microscopy with minimal or no Ig.1
In MGRS-C3GN, injury occurs via dysregulation of the alternative complement pathway, resulting in severe acute kidney injury with poor prognosis.2,6 We report a case of C3GN with no spike in Serum protein electrophoresis (SEP), but positive SIEP. Once the diagnosis was made, the patient was treated with immunosuppression with complete recovery of kidney function. With treatment, the formation of crescents was interrupted and the tubulointerstitial infiltrate was resolved. The treatment killed the immunoglobulin-producing clone. The possibility of recurrence exists. It implies close monitoring of C3 levels and the reappearance of monoclonal gammopathy. This case shows the importance of accurate and prompt diagnosis and appropriate treatment. Monoclonal gammopathy should be considered in adult patients with membranoproliferative glomerulonephritis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
References
- C3 Glomerulonephritis associated with monoclonal gammopathy: A case series. Am J Kidney Dis. 2013;62:506-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Update on C3 glomerulopathy: A complement-mediated disease. Nephron. 2020;144:272-80.
- [CrossRef] [PubMed] [Google Scholar]
- C3 glomerulopathy: Consensus report. Kidney Int. 2013;84:1079-89.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Monoclonal gammopathy of renal significance (MGRS) histopathologic classification, diagnostic workup, and therapeutic options. Neth J Med. 2019;77:243-54.
- [PubMed] [Google Scholar]
- Monoclonal gammopathy of renal significance. N Engl J Med. 2021;384:1931-41.
- [CrossRef] [PubMed] [Google Scholar]
- C3 glomerulonephritis associated with monoclonal gammopathy: A retrospective case series study from a single institute in china. Ren Fail. 2021;43:1437-45.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







