Translate this page into:
Cardiovascular Complications in Kidney Transplant Recipients with COVID-19: A Case Series
Address for correspondence: Ankita Patil, Assistant Professor, Department of Nephrology, Ward No. 34A, Old Building Third Floor, Seth G. S. Medical College and KEM Hospital, Parel, Mumbai - 400 012, Maharashtra, India. E-mail: anki3patil@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Kidney transplant recipients (KTRs) are at a higher risk for developing severe COVID-19 which can be associated with cardiovascular complications. We studied five KTRs recipients infected with COVID-19 who developed severe cardiovascular complications. Two patients presented with ST segment myocardial infarction and two with clinically suspected myocarditis. One patient presented with atrial fibrillation. Two of these patients developed cardiogenic shock. Inflammatory markers were at peak during the event in four of these who had presented with severe COVID-19. Coronary angiography done in two patients with STEMI did not reveal any evidence of atherosclerotic coronary artery disease. Also, based on the cardiovascular (CV) risk estimation by Framingham score, four patients had low CV risk and one patient had intermediate CV risk. All five patients survived. Even with low CV risk, KTRs can develop myocardial injury and arrhythmias solely because of severe COVID-19.
Keywords
Cardiovascular disease
COVID-19
kidney transplant
myocardial injury
Introduction
Increased prevalence of myocardial injury and arrhythmias have been reported in association with COVID-19. Myocardial injury in association with COVID-19 can be both ischemic and nonischemic secondary to a severe inflammatory response syndrome (SIRS) and direct viral cytotoxicity.[1] Acute myocardial injury is defined as an elevation of high-sensitivity cardiac troponin above the 99th percentile of its upper limit of normal and/or evidence of new electrocardiographic and/or echocardiographic abnormalities.[2] Along with the cytokine storm due to severe COVID-19,[3] the presence of traditional risk factors such as hypertension (HTN), diabetes, obesity, dyslipidemia, pre-existing cardiovascular disease, increased recipient age, non-traditional risk factors such as chronic allograft injury, proteinuria, dialysis vintage prior to transplantation, rejections make them vulnerable for the development of severe cardiovascular complications due to COVID-19.[4] Cardiovascular (CV) disease is common in kidney transplant recipients (KTRs) making them susceptible to sudden cardiac death. Hence, early diagnosis, cardiac monitoring and appropriate intervention is essential. There is no available literature yet about details about these severe cardiovascular complications in KTRs.
Case series
Ninety-five KTRs developed COVID-19 from March 2020 till July 2021 at a tertiary care centre in western India. We studied five (5.3%) of these who presented with severe cardiovascular complications. The criteria for diagnosis of clinically suspected myocarditis was one or more of the clinical profiles (acute coronary syndrome-like, new onset or worsening heart failure or a life-threatening arrhythmia or cardiogenic shock) described by the European Society of Cardiology (ESC)[5] with at least one of either raised biomarkers of cardiac injury, electrocardiogram (ECG) findings suggestive of cardiac injury or abnormal cardiac function on echocardiogram (2D echo).[6] Cardiogenic shock was defined as an ineffective cardiac output due to a primary cardiac dysfunction resulting in inadequate end-organ perfusion. Acute allograft dysfunction was defined as an increase in serum creatinine ≥0.3 mg/dl from baseline at diagnosis of COVID-19. Framingham Risk Score[7] was used to estimate the 10-year CV risk of these patients.
The median (IQR) age was 49 (41.5-49.5) years will all five of them being males. All were living-donor related transplant recipients and were on triple or dual immunosuppression at the onset of COVID-19. With respect to presence of traditional risk factors, three out of five had HTN, one had new-onset diabetes after transplant (NODAT) and two had dyslipidemia. Based on the CV risk estimation by Framingham score, four patients had a low CV risk and one patient had an intermediate CV risk. None of them had prior evidence of coronary artery disease (CAD). Two out of five had baseline chronic allograft injury. Four of these had severe COVID-19. Two patients presented with ST segment myocardial infarction (STEMI), two with clinically suspected myocarditis and one with atrial fibrillation. Two of these five developed cardiogenic shock. These manifestations occurred over Day 6- Day 51 post-onset of COVID-19 with Day 0 being the day of diagnosis of COVID-19. Inflammatory markers were at peak at the time of the cardiac event in four of these patients. Acute allograft dysfunction was present in all five patients which recovered at discharge. All five patients survived. Coronary angiography (CAG) was done in two of the patients who presented with myocardial infarction did not show evidence of atherosclerosis. The characteristics of the five patients are summarized in Tables 1 and 2. Their ECG findings are depicted in [Figures 1-3].
| Case | Age/Gender | Severity of COVID-19† | Time of onset post COVID-19‡ | Symptoms | Lymphocyte count at event§ | Inflammatory markers at event¶ | Baseline graft function (mg/dL) | Acute graft dysfunction (mg/dL)α |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 50/M | Severe | Day 15 | Worsening dyspnea | 1825 | IL6 267 | 1.4 | 4.9 |
| IL6 267 LDH 853 CRP 90 D-dimer 2.2 |
||||||||
| Case 2 | 49/M | Severe | Day 9 | Anginaβ | 639 | LDH 2009 | 1.4 | 1.8 |
| CRP 55.8 D-dimer 2.24 |
||||||||
| Case 3 | 49/M | Mild | Day 51 | Anginaβ | NA | NA | 1.3 | 1.6 |
| Case 4 | 48/M | Severe | Day 7 | Anginaβ | 760 | LDH 1332 | 1.63 | 2.3 |
| CRP 34.5 D-dimer 1.20 |
||||||||
| Case 5 | 35/M | Severe | Day 6 | Palpitations | 161 | LDH 484 | 3 | 6.9 |
| CRP 65 D-dimer 0.95 |
||||||||
| Case | Cardiac markers/other investigations | ECG | 2D echo (at diagnosis) | Cardiogenic shockπ | Management | CAG/Intervention | Patient outcome | Renal outcome (serum creatinine in mg/dL at discharge) |
| Case 1 | High sensitivity troponin I 1296 (<14 ng/L) |
Sinus tachycardia T wave inversions in leads V2-V5 and I, aVL |
EF 60%, No RWMA | Yes | Anticoagulation (conventional heparin) Antiplatelets Statin |
No | Alive | 1.6 |
| Case 2 | Fraction of CPK MB out of CPK total: 2% (Normal 3-5%) | ST segment elevation in leads V2-V5, II, III, aVF | EF 40% Anterolateral and septal hypokinesia |
Yes | Fibrinolytic therapy (Streptokinase) Anticoagulation (conventional heparin) Antiplatelets Statin |
Recanalized coronaries No atherosclerotic CAD |
Alive | 1.5 |
| Case 3 | High sensitivity troponin I 13.5 (<14 ng/L) |
ST segment elevation in leads V1-V3 | EF 60%, No RWMA | No | Anticoagulation Antiplatelets Statin |
No atherosclerotic CAD | Alive | 1.3W |
| Case 4 | Fraction of CPK MB out of CPK total=60% (Normal 3-5%) | New ST segment depression and T wave inversion in I, aVL, V4-V6 | EF 60%, No RWMA | No | Anticoagulation Antiplatelets Statin |
Not done | Alive | 1.8 |
| Case 5 | Serum magnesium: 1.1 mg/dL (1.6-2.6) Serum calcium: 8.8 mg/dL (8.5-10.2) Serum potassium: 3.8 meq/L(3.5-5) |
Atrial fibrillation | EF 60%, No RWMA, valves normal | No | Rate control with metoprolol | - | Alive | 4.3 |
†: Severity of COVID-19: Mild: Asymptomatic/Symptomatic infection (upper respiratory infection) with or without comorbidities (>60 years, obesity, diabetes mellitus, hypertension, coronary artery disease, chronic lung disease, chronic kidney disease, immunocompromised state, immunosuppressive drugs) Severe: SpO2(oxygen saturation) < 94 % or requiring oxygen. ‡: Day 0: Day of admission for COVID-19. §: Normal range: 1000-4000 or 20-40%). ¶: Interleukin 6 [IL6 (<7 pg/mL)], Lactate dehydrogenase [LDH (80-300 IU/L)], C-reactive protein, [CRP (<7 mg/L)], D-dimer (<0.5 mg/L). M: Male, F: Female, ECG: Electrocardiogram, 2D echo: Two-dimensional echocardiography, CAG: Coronary angiography, CAD: Coronary artery disease, CPK-MB: Creatine Phosphokinase-Myocardial band, CPK-total: Creatine Phosphokinase Total, MMF: Mycophenolate Mofetil, TAC: Tacrolimus, EF: ejection fraction, RWMA: regional wall motion abnormalities, NA: Not applicable. β: Angina: Chest discomfort thought to be attributable to myocardial ischemia. α: Acute graft dysfunction: Acute allograft dysfunction was defined as increase in serum creatinine ≥0.3 mg/dL from baseline at diagnosis of COVID-19. π: Cardiogenic shock: ineffective cardiac output due to a primary cardiac dysfunction resulting in inadequate end-organ perfusion
| Case | Time post-transplant | BMI (kg/m2) | Donor/relationship | Triple IS at time of transplant | Antibody induction | Dialysis vintage (months) | Native kidney disease | HTN | NODAT | Dyslipidemia† | Pre-transplant history of CAD | Baseline egger‡ (ml/min/1.73 m2) | Baseline proteinuria (per day) | Prior episodes of rejection | Chronic allograft injury§ | 10-year risk of CAD (Framingham risk score)¶ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 8 years | 28.4 | Living donor/wife | TAC/MMF/Steroid | Yes (ATG) | 22 | Not Known | Yes | No | Yes | No | 58.2 | 360 mg | 1) Acute ACR 1m posttransplant treated with ATG 2) Chronic ABMR 6 years posttransplant treated with augmentation of MMF and Tacrolimus |
No | <10% |
| Case 2 | 8 years | 18.3 | Living donor /brother | TAC/MMF/Steroid | Yes (ATG) | 1 | Not Known | No | Yes Duration 8 years 7 months HbA1c 7.5% |
Yes | No | 58.6 | Nil | No | No | 10–20% |
| Case 3 | 5 months | 21.4 | Living donor/wife | TAC/MMF/Steroid | No | 36 | Not Known | No | No | No | No | 64.1 | No | No | No | <10% |
| Case 4 | 7 years | 22.3 | Living donor/wife | CSA/MMF/steroid | Yes | 2 | Not known | Yes | No | No | No | 49.1 | 1.2 gm | 1) Chronic ABMR 2 years posttransplant treated with augmentation of steroids and CSA 2) Clinical suspicion of chronic rejection 6 years posttransplant, inadequate biopsy, CSA changed to TAC |
Yes | <10% |
| Case 5 | 16 years | 18 | Living donor/mother | TAC/ AZA/steroid | No | 4 | Not known | Yes | No | No | No | 25.7 | 2 gm | No | Yes | <10% |
M: Male, F: Female, ATG: Anti-thymocyte Globulin, CAD: coronary artery disease, CV: Cardiovascular, IS: Immunosuppression, HTN: Hypertension, NODAT: New onset diabetes after transplant, TAC: Tacrolimus, CSA: Cyclosporine, AZA: Azathioprine, MMF: Mycophenolate mofetil, ACR: Acute cellular rejection, ABMR: Antibody mediated rejection, BMI: body mass index. †: Dyslipidemia: Elevated plasma total cholesterol, elevated low-density lipoprotein cholesterol (LDL), elevated triglycerides, and/or low high-density lipoprotein cholesterol (HDL) ‡: eGFR (estimated glomerular filtration rate by CKD-EPI -chronic kidney disease epidemiology collaboration. §: Chronic allograft injury: Serum creatinine persisting to be ≥1.5 mg/dL for ≥3 months. ¶: Framingham Risk Score (Cardiovascular risk at 10 years): Low risk ≤10%, Intermediate risk 10-20%, High risk ≥20%.

- Electrocardiogram (ECG) findings of Case 2: ST segment elevation in leads V2–V5, II, III, aVF
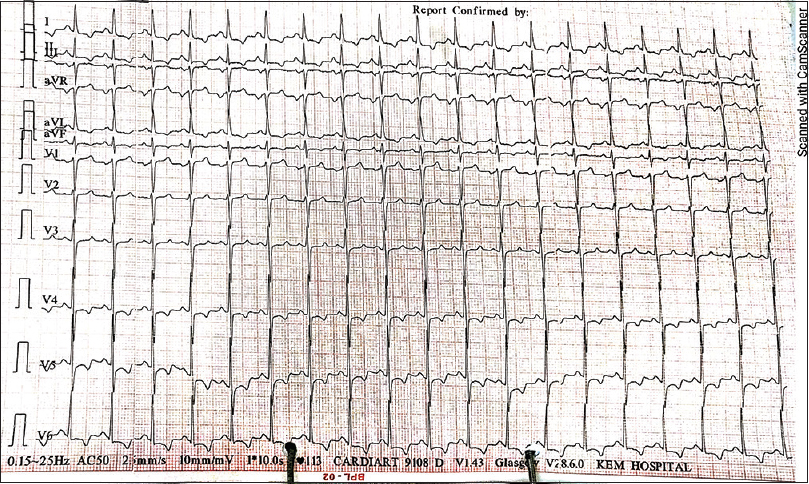
- Electrocardiogram (ECG) findings of Case 4: new ST segment depression and T wave inversion in I, aVL, V4–V6
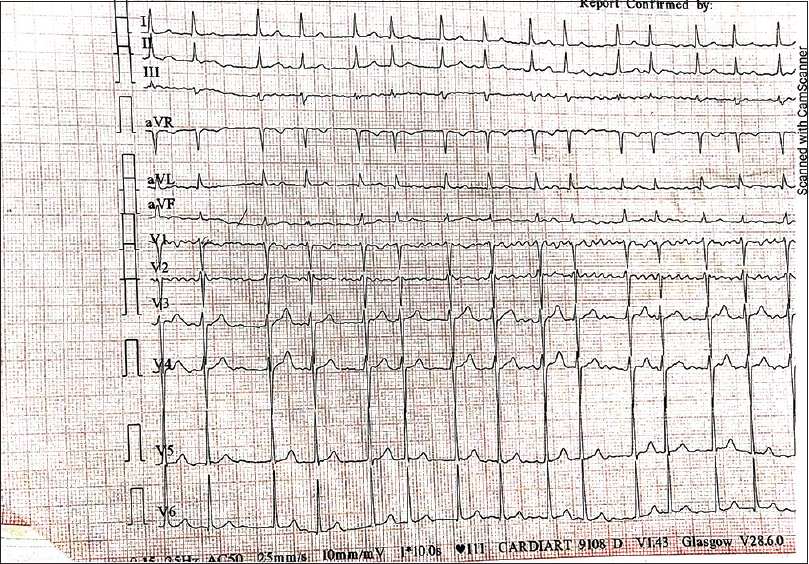
- Electrocardiogram (ECG) findings of Case 5: atrial fibrillation
Discussion
We hereby report a series of five KTRs presenting with severe cardiovascular complications in association with COVID-19. All five patients survived to discharge. To our knowledge, this is the first report of severe cardiovascular complications in KTRs. The prevalence of acute myocardial injury in association with COVID-19 globally has been reported to be 15-38%.[18910] Ischemic myocardial injury due to severe COVID-19 can result either due to plaque rupture, coronary spasm, microthrombi secondary to SIRS, disseminated intravascular coagulation, cytokine storm (Type 1 MI), or due to myocardial oxygen imbalance (Type 2 MI).[1] Myocardial infarction (STEMI) in association with COVID-19 has been reported in total 129 patients as case reports or series.[111213] Most common risk factors in these patients were age >60 years, HTN in 94 (73%), prior CAD in 68 (53%), diabetes mellitus in 50 (38%), chronic kidney disease in 45 (35%), dyslipidemia in 15 (12%), and obesity in 2 (1.6%).[111213] One-hundred and seven (83%) of these had evidence of occlusive CAD and 42 (33%) had peak inflammatory markers at the time of STEMI.[14] Our two patients with ST segment myocardial infarction were younger (mean age 49 years) and had lesser traditional risk factors with no prior history of CAD, suggesting that the ischemic myocardial injury was most probably related to COVID-19. Also, in both our patients with STEMI, CAG did not reveal atherosclerosis, suggesting a COVID-19-related thrombotic event as the most likely cause of myocardial injury. This finding is similar to that reported from the available literature on COVID-19-associated STEMI that a culprit lesion is not identifiable by coronary angiography in 40% of patients.[14] Both these patients had peak inflammatory markers at the time of myocardial infarction.
Acute myocarditis with COVID-19 has been reported in 86 patients as case reports or series.[15161718] The median age was 48 years in these patients with inflammatory markers being at peak in 35 (64%) at the time of myocarditis.[15161718] Direct viral cytotoxicity along with an inflammatory cascade is said to be responsible for nonischemic injury such as acute myocarditis.[1] Both our cases with clinically suspected myocarditis were of a similar age (mean 49 years) with peak inflammatory markers at the time of the event. The prevalence of atrial fibrillation in association with severe COVID-19 has been reported to be 19%,[19] especially in ages ≥60 years. Hypoxia and electrolyte abnormalities during severe COVID-19 contribute to the development of acute arrhythmias.[19] Also, it has been suggested that the SARS-CoV-2 virus directly contributes to atrial fibrillation by attaching to pericytes, cells responsible for microvascular integrity of cardiac tissue resulting in the release of growth factors, causing cardiac tissue inflammation and altering atrial cellular electrophysiology.[20] Our patient who had a new-onset atrial fibrillation was younger (35-year-old), had no prior CAD or valvular heart disease, and had presented with severe COVID-19. From the available literature, myocardial injury was reported to be coinciding with peak levels of inflammatory markers (C-reactive protein, lactate dehydrogenase, D-dimer, interleukin 6) and lymphopenia which was evident in four of our patients.[1415161718] In our series, these manifestations occurred over day 6–day 51 post-onset of COVID-19 with day 0 being the day of diagnosis of COVID-19 as compared to that reported in the literature to be over day 0–day 30.[1112131415161718] Development of CV complications in association with COVID-19 has been shown to significantly increase mortality.[21] All five of our patients survived. They were treated in dedicated high dependency renal units with close cardiac monitoring. As four of our patients had low CV risk and one had an intermediate risk (Framingham risk score), it is very likely that these events were related to COVID-19. Retrospective data collection and the absence of CMR/myocardial biopsy along with coronary angiography in those with clinically suspected myocarditis remain notable limitations.
To conclude, even with low CV risk, KTRs can develop both ischemic and nonischemic myocardial injury and arrhythmias solely because of severe COVID-19. A high index of suspicion, cardiac monitoring, and prompt management is essential to prevent mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054-62.
- [Google Scholar]
- Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231-64.
- [Google Scholar]
- A systematic review of COVID-19 infection in kidney transplant recipients: A universal effort to preserve patients' lives and allografts. J Clin Med. 2020;9:2986.
- [CrossRef] [Google Scholar]
- Risk factors for cardiovascular events after successful renal transplantation. Transplantation. 2008;85:209-216.
- [Google Scholar]
- Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2013;34:2636-48.
- [Google Scholar]
- General cardiovascular risk profile for use in primary care. Circulation. 2008;117:743-53.
- [Google Scholar]
- The impact of 2019 novel coronavirus on heart injury: A systematic review and meta-analysis. Prog Cardiovasc Dis. 2020;63:518-24.
- [Google Scholar]
- Cardiac injury and COVID-19: A systematic review and meta-analysis. CJC Open. 2020;2:386-94.
- [Google Scholar]
- Acute cardiac injury in COVID-19: A systematic review and meta-analysis. Arch Iran Med. 2020;23:801-12.
- [Google Scholar]
- Acute myocardial infarction secondary to COVID-19 infection: A case report and review of the literature. Clin Imaging. 2021;72:178-82.
- [Google Scholar]
- ST-segment elevation in patients with covid-19 — A case series. N Engl J Med. 2020;382:2478-80.
- [Google Scholar]
- Characteristics and outcomes in patients presenting with COVID-19 and ST-segment elevation myocardial infarction. Am J Cardiol. 2020;131:1-6.
- [CrossRef] [Google Scholar]
- Case report of a COVID-19-associated myocardial infarction with no obstructive coronary arteries: The mystery of the phantom embolus or local endothelitis. Eur Heart J Case Rep. 2021;5:ytaa521.
- [CrossRef] [Google Scholar]
- Acute myocarditis in COVID19 patients. Clinical features, severity and outcomes. Results from Spanish multicenter registry car-COVID19. Eur Heart J Acute Cardiovasc Care. 2021;10(Suppl 1):zuab020-188.
- [Google Scholar]
- Myocarditis associated with Covid-19 disease: A systematic review of published case reports and case series. Int J Clin Practice. 2021;75:e14470.
- [CrossRef] [Google Scholar]
- Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm. 2021;18:501-7.
- [Google Scholar]
- Incidence and mortality risk in coronavirus disease 2019 patients complicated by acute cardiac injury: Systematic review and meta-analysis. J Cardiovasc Med. 2020;21:759-64.
- [Google Scholar]







