Translate this page into:
Cerebral venous thrombosis in a patient with acute postinfectious glomerulonephritis
Address for correspondence: Dr. P. S. Priyamvada, Department of Nephrology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry - 605 006, India. E-mail: priyamvadaps@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Thrombosis of the cerebral venous sinuses (CVT) is described in nephrotic syndrome. A 13-year-old girl was admitted with acute post-infectious glomerulonephritis (APIGN). Subsequently she developed recurrent seizures with focal neurological deficits. On evaluation, she was found to have CVT. To the best of our knowledge, this is the first report of CVT in APIGN. Identifying this complication is imperative, as timely diagnosis and treatment could be lifesaving.
Keywords
Acute nephritic syndrome
seizure
thrombosis
Introduction
Thrombosis of the cerebral venous sinuses (CVT) has been described in adults and children with nephrotic syndrome.[12] Acute post-infectious glomerulonephritis (APIGN), the prototype of acute nephritic syndrome is not reported to increase the risk for venous thrombosis. Here we report a case of CVT in a patient with APIGN.
Case Report
A 13-year-old girl presented with recent onset generalized edema, decreased urine output, high-colored urine, and persistent vomiting. Two weeks ago she had fever and cough, which was successfully treated with a short course of antibiotics. On admission her blood pressure was 160/100 mm of Hg, urine showed 2+ protein and dysmorphic red blood cells. The other relevant investigations are shown in Table 1. Ultrasound abdomen revealed normal sized kidneys. She was treated with anti-hypertensives and diuretics. Over next 3 days, her blood pressure came down and urine output improved, but serum creatinine increased to 5.1 mg/dl. Hence, she was started on intravenous methylprednisolone injections at a dose of 750 mg/day for 3 days, followed by oral prednisolone at 1 mg/kg.

On the 5th day after initiation of steroid therapy, she developed recurrent episodes of generalized tonic-clonic seizures associated with altered sensorium. Her blood pressure at the time of seizures was 130/80 mm of Hg. On examination, she was found to have left sided hemiparesis. Non-enhanced computed tomography (CT) brain showed an infarct in the left temporo-parietal region, mild midline shift, and cerebral edema. Hyperdensities were observed in the sagittal sinus, right sigmoid, and transverse sinuses [Figure 1a]. A CT venogram showed an empty delta sign with filling defects in right transverse and sigmoid sinus extending to the right internal jugular vein [Figures 1b, 2a and b]. She was started on anticoagulation with continuous infusion of unfractionated heparin (UFH). Hemodialysis was initiated through right femoral catheter in view of persistent renal failure. The seizures were controlled and sensorium improved over the next 1-week. Heparin was switched over to warfarin at the end of 7 days. She was supported with hemodialysis for 1-week, subsequently her renal function started to improve. After the initial decline, the serum creatinine remained static at 4.8 mg/dl. Her ANA, lupus anticoagulant (LA), anticardiolipin antibody, and ANCA were negative. We could not proceed with thrombophilia work up due to financial constraints.
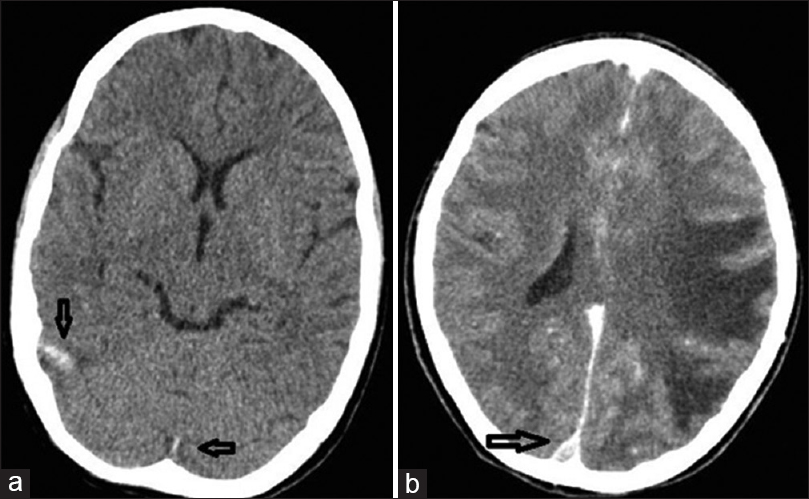
- (a) Nonenhanced computed tomography brain showing thrombosed cortical veins (Dense clot sign), (b) Computed tomography venogram showing empty delta sign suggestive of superior sagittal sinus thrombosis (arrows). The computed tomography also shows extensive infarction of left temporal and parietal lobes
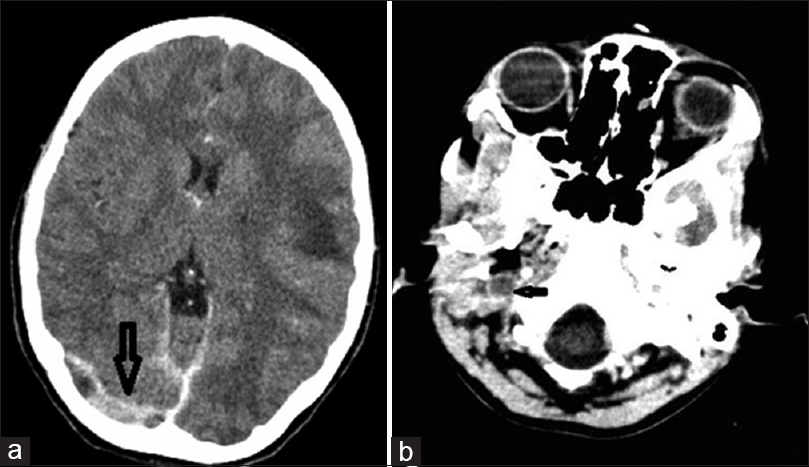
- (a) Computed tomography venogram showing thrombosis of the right transverse sinus (arrows), (b) Computed tomography venogram showing thrombosis of the right internal jugular vein (arrows)
Renal biopsy revealed enlarged glomeruli showing endocapillary proliferation, with neutrophils and occasional eosinophils in the capillary lumina. Glomerular basement membrane (GBM) thickness was normal. A segmental cellular crescent was present in one glomerulus. Tubules, interstitium, and vessels were normal. Immunofluorescence microscopy (IF) showed diffuse granular deposits of IgG and C3 (3+ intensity) along the capillary loops. Tubules showed simplification of the lining epithelium. Interstitium and vessels were unremarkable. The renal biopsy was consistent with post-infectious glomerulonephritis [Figure 3a–d].
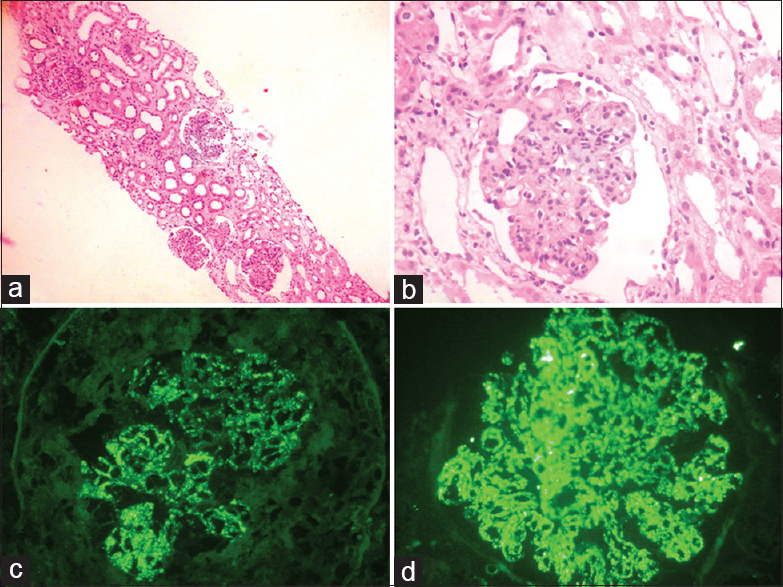
- (a) Glomeruli with marked endocapillary proliferation and simplification of tubular lining epithelium (H and E, ×100), (b) Glomeruli shows marked endocapillary proliferation and neutrophil infiltration occluding the capillary lumina of glomerulus (H and E, ×200), (c and d) Immunofluorescence showing strong diffuse granular coarse deposits of IgG and C3c along glomerular basement membrane
Even though the biopsy was suggestive of post-infectious glomerulonephritis, we decided to continue corticosteroids in view of incomplete recovery of renal function. The patient was discharged on prednisolone 40 mg/day, warfarin and antiepileptics. Over the next 6 weeks, her serum creatinine decreased to 1 mg/dl. Her erythrocyte sedimentation rate decreased to 20 mm/1st h. Prednisolone was given for a total duration of 3 months. Anti-epileptics and anticoagulants were stopped after 6 months. Currently, the patient is off anticoagulation for the last 8 months; without any recurrent episodes of thrombosis. On last follow-up, her blood pressure was 120/80 mm of Hg, serum creatinine 0.8 mg/dl, and 24 h urine protein was <150 mg/dl with normal urine sediment.
Discussion
CVT is considered to be less common when compared to thrombosis of other vascular beds. Apart from a few case series and single case reports, there are no reliable estimates on the prevalence of CVT in glomerular diseases. CVT has been described in patients with idiopathic nephrotic syndrome, minimal change disease, and membranous nephropathy.[12] Nephrotic syndrome induces a hypercoagulable state due to increased blood viscosity, elevated levels of procoagulant proteins like fibrinogen, factor V, VIII, and X, and deficiency of coagulation inhibitors like antithrombin III, protein S and C. CVT is reported to occur at the onset of nephrotic syndrome as well as during relapses. A poorly controlled nephrotic state, as well as steroid resistance confer a high risk for the development of CVT.
Apart from disease related factors, drugs can also trigger venous thrombosis. The volume depletion induced by aggressive diuretic therapy can trigger thrombosis in individuals with significant proteinuria. Exogenous corticosteroid administration is associated with increased thromboembolic tendencies in the initial days of exposure.[3] There have been reports of high dose corticosteroid therapy predisposing to CVT in patients with multiple sclerosis with no additional risk factors for thrombosis.[4] The proposed mechanisms include increased release of von Willebrand factor, shortened prothrombin time (PT) and impaired fibrinolytic activity.
The association between PIGN and CVT does not appear to be direct. The patient had hypoalbuminemia and elevated lipid levels suggestive of a nephrotic state. The coexisting acute tubular necrosis would have been responsible for the lower rates of urinary protein excretion. The volume depletion induced by loop diuretics in conjunction with low serum albumin levels, elevated lipids, and high dose corticosteroids might have precipitated CVT in this patient. There was no evidence of SLE or antiphospholipid antibodies. We did not test for deficiencies of protein C, protein S, antithrombin III, factor V, and prothrombin gene mutations due to financial constraints. The current evidence does not recommend routine thrombophilia testing in all patients with first episode of CVT as it has a low value for predicting recurrences.[567] It is possible that the patient might be having a preexisting mild thrombophilia, which in the presence of appropriate triggers might have precipitated CVT.
Seizure is not an uncommon complication of PIGN. The most common cause of seizures in PIGN is hypertensive encephalopathy. There have been few reports of posterior reversible encephalopathy syndrome causing recurrent seizures in PIGN. Since CVT can also present as seizures, a high index of suspicion and appropriate early investigations are required to identify this entity.
Conclusions
We wanted to highlight the occurrence of CVT in a patient with APIGN, a disease which is not usually associated with a hypercoagulable state. The nephrotic state resulting from APIGN combined with the volume depletion would have precipitated CVT in this patient. It is possible that CVT might be underdiagnosed because of the wide variability in clinical presentation. Recognizing this rare complication is important because of the potentially devastating outcome, if left untreated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cerebral venous sinus thrombosis in adult nephrotic syndrome. Clin Nephrol. 2010;74:144-9.
- [Google Scholar]
- Cerebral venous thrombosis as a complication of nephrotic syndrome – A case report and literature review. Rinsho Shinkeigaku. 2014;54:495-501.
- [Google Scholar]
- Use of oral glucocorticoids and the risk of pulmonary embolism: A population-based case-control study. Chest. 2013;143:1337-42.
- [Google Scholar]
- High-dose corticosteroid treatment is associated with an increased risk of developing cerebral venous thrombosis. Hippokratia. 2013;17:88-90.
- [Google Scholar]
- Cerebral venous sinus thrombosis: Update on diagnosis and management. Curr Cardiol Rep. 2014;16:523.
- [Google Scholar]
- Guidelines on the investigation and management of venous thrombosis at unusual sites. Br J Haematol. 2012;159:28-38.
- [Google Scholar]
- Cerebral venous thrombosis and thrombophilia: A systematic review and meta-analysis. Semin Thromb Hemost. 2013;39:913-27.
- [Google Scholar]







