Translate this page into:
Coexistent Amyloid Fibrils in a Patient with Combined Light Chain Deposition Disease and Light Chain Cast Nephropathy
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Monoclonal secretion of light chains can affect the kidney with varied morphologic manifestations. Myeloma cast nephropathy, proximal tubulopathies, monoclonal immunoglobulin deposition disease, amyloidosis, and tubulointerstitial nephritis are often noted in the renal biopsy of these patients. Most often the histopathological manifestations affect only one compartment of the nephron (tubules, capillaries, glomerulus). Case reports of a combination of cast nephropathy and monoclonal light chain deposition disease are also reported in literature. Here we report an unusual case of coexisting cast nephropathy, light chain deposition disease, and deposition of amyloid fibrils in the biopsy of a lady with monoclonal kappa light chain excretion.
Keywords
Amyloid fibrils
cast nephropathy
light chain deposition disease
Introduction
Plasma cell dyscrasias are known to affect the kidneys and can present with different morphologic patterns. In most cases, usually one pattern of renal pathology is seen. However, a combination of patterns occurring in the same patient have been documented. The incidence of renal light chain deposition disease (LCDD) with coexistent cast nephropathy (CN) has been reported in literature. This combination of pathology in the renal biopsy is considered as the most common pattern. We report a unique case of a woman who presented to us with progressive renal failure and was subsequently diagnosed on renal biopsy to have three morphological pathologies: CN, LCDD, and deposition of amyloid fibrils at the glomerular hilum.
Case Report
A 65-year-old woman presented to our outpatient department with complaints of generalized weakness and low backache for 6 months. She also gave a history of nausea, vomiting, and loss of appetite for the past 2 weeks. The patient gave history of fracture of right tibia after she had an injury to her right leg following a trivial injury, for which an open reduction and internal fixation was done on the affected fracture leg. She denied any history of decreased urine output, breathing difficulty, weight loss, hair loss, oral ulcers, bone and joint pains, rash, and hematuria. Serum creatinine at our hospital was 6.8 mg/dL. The patient was then admitted for further management.
On clinical evaluation, her vitals were found to be normal. She had pallor, and there was no icterus or pedal edema. Systemic examination showed no significant clinical findings. Her electrolytes and liver function tests were normal. Her spot urine protein creatinine ratio was 1.2. Ultrasound showed normal sized kidney with normal echotexture. Anti-ds DNA, antinuclear antibody profile, cytoplasmic antineutrophil cytoplasmic antibodies, perinuclear anti-neutrophil cytoplasmic antibodies, complements C3 and C4, anti-glomerular basement membrane antibody tests were all negative. Her renal functions deteriorated further and she was initiated on hemodialysis. A renal biopsy was performed.
Renal biopsy showed a mild mesangial expansion of the glomeruli with few showing ischemic changes. Few of the tubules contained fractured casts, which were pale staining with periodic acid Schiff (PAS) stain with associated histiocytic reaction consistent with CN [Figure 1]. On immunofluorescence, there was a strong staining for Kappa light chain (3+) in the fractured casts and tubular basement membranes, whereas staining for Lambda light chain was negative [Figure 2a and b]. Staining for IgG, IgA, IgM, C3, and C1q was negative. No glomerular deposits were noted. Electron microscopy showed deeply osmiophilic flocculent material within the tubular lumina [Figure 3a]. Fine granular powdery deposits were also seen along tubular basement membranes [Figure 3b]. Hilar region of a single glomerulus also showed aggregates of pale non-branching fibrils measuring 8–11 nm in diameter [Figure 3c]. Based on light microscopy, immunofluorescence, and electron microscopy, the final pathological diagnoses included (i) CN, (ii) LCDD, and (iii) presence of amyloid fibrils at the glomerular hilum.

- Fractured tubular casts with focal histiocytic reaction surrounding the casts
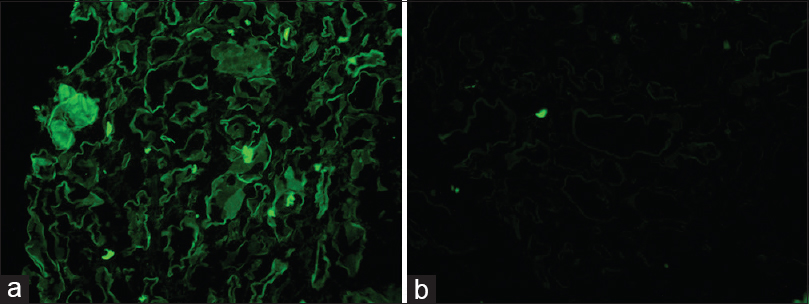
- (a) Strong positive staining for Kappa light chain seen in the tubular basement membranes and in the fractured cast. (b) There is no staining for Lambda light chain around the tubules or the casts
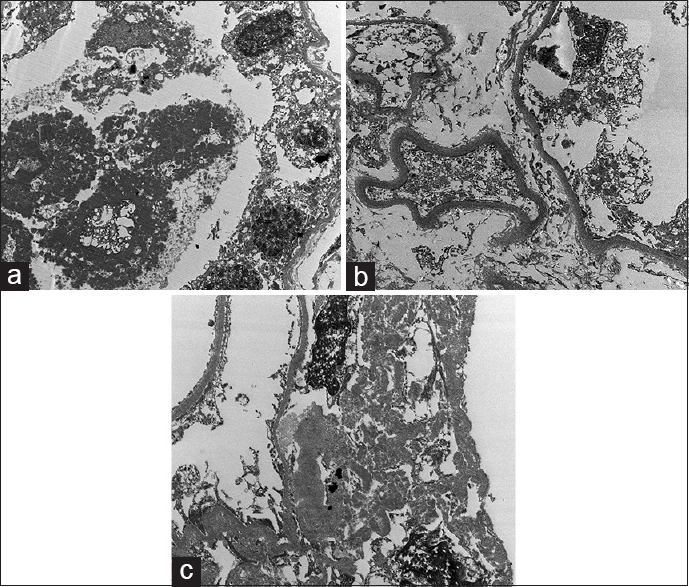
- (a) Deeply osmiophilic coarse granular casts within tubules. (b) Powdery deposits along tubular basement membrane. (c) Aggregates of pale non branching fibrils measuring 8 to 11 nm in diameter in the hilum of the glomerulus
Further investigations revealed no M band on serum and urine protein electrophoresis. Quantitative immunofixation revealed an abnormal excretion of kappa light chains (643 mg/dL, normal range 3.3–19.4 mg/dL) and an altered free kappa/lambda chain ratio of 26.68 (ratio in renal impairment 0.37–3.1). Bone marrow aspiration and biopsy showed 8% polyclonal plasma cells. There were no lytic lesions in the skeletal survey. The patient was started on treatment with once-weekly regimen of injection bortezomib, dexamethasone, and cyclophosphamide. Six months later, she continues on thrice-weekly hemodialysis with no clinical improvement in her renal condition.
Discussion
We present here a rare and previously unreported case of monoclonal gammopathy of renal significance (MGRS) associated with a combination of three pathologies – light chain CN, LCDD, and deposition of amyloid fibrils. This pathological picture on renal biopsy was secondary to monoclonal excretion of kappa light chains. Kidney is one of the most commonly affected organ in patients with MGRS and renal biopsy is often that of monoclonal immunoglobulin deposition disease (MIDD).[12] Our patient did not have the classical PAS-positive globular nodular mesangial sclerosis. In fact, the glomeruli were almost normal with mild mesangial widening. Typical fractured casts were seen in histopathology. Immunoflourescence revealed a strong staining of kappa light chain on the tubular basement membrane and on fractured casts. Histopathologically and on immunofluorescence, our case was a combination of CN and LCDD (kappa light chain restricted). This is in accordance with the findings of Lin et al. who reported combined cases of MIDD and CN. They showed that the pathology differed significantly in appearance from those of pure MIDD.[1] In their series, only 2 of 11 cases of combined LCDD and CN showed typical features of nodular sclerosing glomerulopathy, whereas the others showed only a mild mesangial expansion or appeared histologically normal. In another series, glomeruli were histopathologically normal in 69% patients;, however linear light chain reactivity was seen in 100% of these biopsies.[3] Similar combination of LCDD and CN has been reported in the past decade in other studies also.[45]
The electron microscopy in our patient showed the presence of deeply osmiophilic flocculent material within the tubular lumina accompanied by fibrillary aggregates (mean fibril diameter of 8–11 nm) in the glomerular hilum. Fine granular powdery deposits were also seen along tubular basement membranes consistent with kappa light chain deposition as was noted in immunofluorescence. This led to the addition of the presence of amyloid fibrils in the final pathological diagnosis. This unusual pathological presentation is rare and only a couple of cases of similar nature have been reported in literature. Qian et al. reported a case with all the three pathologies; however, their case had an established diagnosis of multiple myeloma, had monotypic Ig λ in immunofluorescence and the fibrils were of 19.65 nm in diameter. The fibrils were also more extensively deposited in the glomeruli.[6] Another case reported in literature had all the combined pathology with amyloid staining in the interstitium and the arterial wall.[7] In our case, this unusual ultrastructure of light chains (powdery granular deposits around the tubules and non-branching fibrils in the hilum of the glomerulus) highlight the variable morphologic manifestation of the paraprotein.
The pathophysiologic basis of light chains producing various morphologic manifestations is not yet clear, but it is suggested that the variable physicochemical characteristics of the light chain and the renal microenvironment determine the morphology.[89] Our case with a unique presentation adds to the burgeoning literature on myriad renal morphology in patients presenting with monoclonal paraprotein excretion and perhaps will help in understanding the pathophysiology of monoclonal light chain damage of the kidneys.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Renal monoclonal immunoglobulin deposition disease: The disease spectrum. J Am Soc Nephrol. 2001;12:1482-92.
- [Google Scholar]
- Renal monoclonal immunoglobulin deposition disease: A report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7:231-9.
- [Google Scholar]
- Morphologic manifestations of combined light chain deposition disease and light-chain cast nephropathy. Ultrastruct Pathol. 2007;31:141-9.
- [Google Scholar]
- Monoclonal gammopathy: Significance and possible causality in renal disease. Am J Kidney Dis. 2003;42:87-95.
- [Google Scholar]
- Light chain deposition disease of the kidney: Morphological aspects in 24 patients. Virchows Arch. 1994;425:271-80.
- [Google Scholar]
- Coexistence of myeloma cast nephropathy, light chain deposition disease and non amyloid fibrils in a patient with multiple myeloma. Am J Kidney Dis. 2010;56:971-6.
- [Google Scholar]
- Renal failure due to combined cast nephropathy, amyloidosis and light chain deposition disease. Nephrol Dial Transplant. 2010;25:1340-3.
- [Google Scholar]
- Mechanisms of disease: Monoclonal immunoglobulin deposition. Amyloidosis, light chain deposition disease, and light and heavy chain deposition disease. Hematol Oncol Clin North Am. 1992;6:323-46.
- [Google Scholar]







