Translate this page into:
Comparative Analysis of Determinants and Outcome of Early and Late Acute Antibody Mediated Rejection (ABMR)
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Antibody-mediated rejection (ABMR) is one of the major determinants of graft survival. Although diagnostic precision and treatment options have improved, response to therapy and graft survival has not improved very significantly. The phenotypes of early and late acute ABMR differ in many ways. In this study, we assessed the clinical characteristics, response to therapy, DSA positivity, and outcomes of early and late ABMR.
Methods:
During the study period, 69 patients with acute ABMR diagnosed on renal graft histopathology were included with a median follow-up of 10 months after rejection. Recipients were stratified into early acute ABMR (<3 months of transplant; n = 29) and late acute ABMR (>3 months of transplant; n = 40). Graft survival, patient survival, response to therapy, and doubling of serum creatinine were assessed and compared between the two groups.
Results:
Baseline characteristics and immunosuppression protocols were comparable between the early and late ABMR groups. Late acute ABMR had an increased risk of doubling of serum creatinine than the early ABMR group (P = 0.002). Graft and patient survival were not statistically different between the two groups. Response to therapy was inferior in the late acute ABMR group (P = 0.00). Pretransplant DSA was present in 27.6% in the early ABMR group. Late acute ABMR was frequently associated with nonadherence or suboptimal immunosuppression and low DSA positivity (15%). Infections such as CMV, bacterial, and fungal infections were similar in the earlier and late ABMR groups.
Conclusion:
Late acute ABMR group had a poor response to anti-rejection therapy and also an increased risk of doubling of serum creatinine compared to the early acute ABMR group. There was also a tendency toward increased graft loss in late acute ABMR patients. Late acute ABMR patients are more frequently associated with nonadherence/suboptimal immunosuppression. There was also a low incidence of anti-HLA DSA positivity in late ABMR.
Keywords
ABMR
DSA
doubling of creatinine
graft survival
nonadherence
plasmapheresis
Introduction
In recent years, acute antibody-mediated rejection is recognized as one of the major determinants of graft survival. With the advent of calcineurin inhibitors and other effective immunosuppression, the incidence of severe acute cellular rejection has decreased,[1] but ABMR has been recognized more commonly. In recent years, the understanding of the pathogenesis of ABMR and diagnostic criteria for ABMR had undergone several revisions. With the introduction of C4d and donor-specific antibodies (DSA) in clinical practice, the recognition of ABMR has increased in recent years. The update of the Banff classification in 2013 has given a definition of C4d negative ABMR.[2] Although the diagnostic tools and recognition of ABMR have become more objective, the treatment, response to therapy, graft, and patient survival following treatment of ABMR have not shown a significant improvement.[3] Conventional therapies such as plasma exchange and intravenous immunoglobulin (IVIG) have been primarily used in the treatment of ABMR.[4] Other novel therapies such as rituximab and bortezomib[5] have also been tried. Randomized trials that used rituximab along with conventional therapies have not shown significant graft survival benefits.[6]
The Banff meeting report of 2011 differentiated ABMR into two phenotypes:[7] phenotype-1, which develops mainly in presensitized patients leading to early acute ABMR, and phenotype-2, which occurs late post-transplantation when Denovo DSA develops. Studies mention that late acute ABMR has different pathogenesis than early acute ABMR. Over the past years, various studies have demonstrated that late acute rejection has a poor response to therapy and a negative impact on renal allograft survival than early acute rejection episodes.[5,8-10] This study aimed to compare the clinical characteristics, determinants, and outcomes of early and late acute ABMR.
Materials and Methods
All renal transplant recipients with biopsy-proven ABMR between March 2009 and September 2016 were included in this study and followed till December 2016. Biopsies with chronic ABMR changes and ABO-incompatible transplant recipients were excluded. ABMR was divided into two groups based on the timing of rejection. Early acute ABMR was defined as rejection within 3 months of renal transplant and late acute ABMR as >3 months after transplant. From March 2009 to September 2016, 869 renal transplants have been done in our institute. Among these, 69 patients had 82 episodes of acute ABMR. Data were collected retrospectively from the hospital information system. Follow-up time was defined as the time from acute ABMR to death or the end of the study.
ABMR definition
The graft biopsy was analyzed by the same pathologist in our institute. Biopsies were graded according to Banff 97 classification with the 2013 update.
Acute ABMR: All three features must be present for diagnosis of ABMR
-
Morphological evidence (at least one of the following): neutrophils and/or monocytes/macrophages in peritubular capillaries and/or glomeruli (peritubular capillaritis, glomerulitis); arterial fibrinoid necrosis thrombi in glomerular capillaries, arterioles and/or small arteries; acute tubular injury without other apparent causes
-
Immunohistological evidence (at least one of the following): diffuse C4d in peritubular capillaries by IF on frozen sections; diffuse or focal C4d in peritubular capillaries by IHC on paraffin sections; immunoglobulin and/or complement in arterial fibrinoid necrosis
-
Serologic evidence of circulating antibodies to donor HLA or other specific DSA.
The diagnosis of acute ABMR was based on the abovementioned Banff 2013 update. Biopsies meeting criterion 1 and either criterion 2 or 3 but not both for acute ABMR were designated as suspicious for acute ABMR.
Donor-specific antibody single antigen bead assay was performed on Luminex platform using standard technique. Cutoff value of mean fluorescence intensity (MFI) > 1000 in our lab was taken as positive for the presence of donor-specific antibodies. All patients had negative CDC and flow cytometry test crossmatch at the time of transplant. HLA typing was done using the sequence-specific oligonucleotide (MFI) molecular method on the Luminex platform. Baseline characteristics, including patient’s age, sex, comorbidities, donor age, donor sex, relation, HLA matching, pretransplant DSA, type of induction, type of immunosuppressive drugs, adherence to regimen, number of rejection episodes, and baseline serum creatinine, were collected.
Acute rejections were treated with IV methylprednisolone 500 mg 3–5 doses. The treatment of acute ABMR includes plasmapheresis and intravenous immunoglobulin (IVIG). Most patients got five sessions of plasmapheresis and IVIG 400 mg/kg (in 5 doses). In some cases, rituximab 375 mg/m2 was given as 1–2 doses. Response to treatment was defined as the return of serum creatinine to the level of less than or equal to 25% of baseline serum creatinine levels. Doubling of serum creatinine, doubling time, graft loss, and death were correlated with patient and graft survival outcomes. Causes of death and serious infections were recorded. Risk factors for early and late ABMR and response to plasmapheresis were analyzed.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software. Continuous variables were reported as mean (SD) and median (total range). Categorical variables were reported as frequencies. Groups were compared using Mann–Whitney test, independent samples t test for continuous variables, and Chi-square test for categorical variables. Survival analysis was performed using the Kaplan-Meier method using the log-rank test for significance.
Patient survival was calculated from the time of rejection to death or end of the study. Graft survival was calculated from the time of rejection to graft loss (censored for death) or study end. Survival plots were also derived for comparing the doubling of serum creatinine between two groups.
Results
During the study period, we identified 69 patients with biopsy features suggestive of acute ABMR. Baseline characteristics [Table 1] such as recipient age and sex, donor age and sex, and HLA matching were not significantly different between the two groups. There was no significant difference between the two groups in terms of induction therapy and maintenance immunosuppressive therapy [Table 2]. The mean tacrolimus levels in the early and late acute ABMR group were 8.0 ± 1.1 and 3.9 ± 1.1 ng/ml, respectively, and the mean cyclosporine (C0) levels were 400.0 ± 70.7 and 124.5 ± 44.7 ng/ml, respectively. In the late acute ABMR group with a history of drug default, the mean tacrolimus and C0 levels were 2.7 ± 0.7 and 65.0 ± 10.0 ng/ml, respectively. Combined type of rejection was present in nine patients in the late acute ABMR group compared to five patients in the early group (P = 0.59) [Table 3].
| Characteristics | Early acute ABMR (n=29) | Late acute ABMR (n=40) | P |
|---|---|---|---|
| Age (mean±SD) | 35.1+9.8 | 37.8+11.8 | 0.31 |
| Recipient sex, male n (%) | 23 (79.3%) | 36 (90%) | 0.21 |
| Donor age (years) | 45.4±11.1 | 46.5±12.3 | 0.70 |
| Donor gender, female, n (%) | 20 (68.9%) | 29 (72.5%) | 0.75 |
| Time on dialysis, duration in months | 17.8±12.8 | 12.3±10.5 | 0.06 |
| HLA match | |||
| A match ≥1 | 20 (68.9%) | 34 (85%) | 0.11 |
| B match ≥1 | 20 (68.9%) | 30 (75%) | 0.61 |
| DR match ≥1 | 20 (68.9%) | 28 (70%) | 0.97 |
| Diabetes | 0 | 4 (10%) | 0.07 |
| Donor | |||
| Related | 17 (58.6%) | 24 (60%) | |
| Spousal | 11 (37.9%) | 13 (32.5%) | |
| Unrelated | 0 | 0 | |
| Cadaveric | 1 (3.4%) | 3 (7.5%) | |
| Delayed graft function | 7 (24.1%) | 2 (5%) | 0.05 |
| Characteristics | Early ABMR (n=29) | Late ABMR (n=40) | P |
|---|---|---|---|
| Induction | |||
| ATG | 7 (24.1%) | 12 (30%) | NS |
| Basiliximab | 19 (65.5%) | 19 (47.5%) | |
| None | 3 (10.3%) | 9 (22.5%) | |
| CNI | |||
| Cyclosporine | 2 (6.8%) | 10 (25%) | NS |
| Tacrolimus | 27 (93.1%) | 30 (75%) | |
| Antiproliferative drugs | NS | ||
| Azathioprine | 0 | 2 (5%) | |
| Mycophenolate | 28 (96.5%) | 38 (95%) | |
| Everolimus | 1 (3.4%) | 0 | |
| Drug default/suboptimal IS | 1 (3.4%) | 12 (30%) | 0.00 |
| Time to rejection (in days) | 8 (median) | 482.4 | 0.00 |
| Number of patients with positive Pretransplant DSA | 4 (13.7%) | 1 (2.5%) | 0.00 |
| Number of patients who underwent Denovo DSA test | 14 (48.2%) | 20 (50%) | |
| Number of patients with positive Denovo DSA | 4 (13.7%) | 5 (12.5%) | 0.96 |
| Follow-up period after rejection (in months) | 10 (median) | 10 | 0.918 |
| Characteristics | Early ABMR (n=29) | Late ABMR (n=40) | P |
|---|---|---|---|
| Type of rejection | |||
| Combined | 5 (17.2%) | 9 (22.5%) | 0.59 |
| C4d positivity | 19 (65.5%) | 35 (87.5%) | 0.06 |
Pretransplant DSA was positive in four patients in the early group compared to only one patient in the late group (P = 0.01). Denovo posttransplant DSA by single antigen bead assay (SAB) on the Luminex platform was done in 14 (48.2%) and 20 (50%) patients in early and late acute ABMR groups, respectively. DSA was found to be positive in four and five patients in the early and late ABMR groups, respectively.
The median time to ABMR in the early group was 8 days and in the late ABMR group was 482.4 days, which was statistically significant (P = 0.05). All the patients received plasmapheresis and IVIG. The median number of sessions was five. Some patients who were refractory to treatment also received rituximab (n = 4) and bortezomib (n = 1). The median follow-up time after ABMR in both groups was 10 months (P = 0.91).
Response to treatment was seen in 28 out of 29 patients in the early acute ABMR group and in 28 out of 40 patients in the late acute ABMR group, which was statistically significant (P = 0.01) [Table 4].
| Characteristics | Early ABMR (n=29) | Late ABMR (n=40) | P |
|---|---|---|---|
| Response to ABMR treatment | 28 (96.5%) | 28 (70%) | 0.00 |
| Doubling of S. creatinine | 3 (10.3%) | 12 (30%) | 0.00 |
| Graft failure | 1 (3.4%) | 6 (15%) | 0.11 |
| Death | 3 (10.3%) | 3 (7.5%) | 0.67 |
| All infection | 14 (48.2%) | 17 (42.5%) | 0.63 |
| CMV infection | 2 (6.8%) | 3 (7.5%) | 0.92 |
| Fungal infection | 2 (6.8%) | 3 (7.5%) | 0.92 |
One patient developed graft failure in the early acute ABMR group and six patients in the late acute ABMR group (P = 0.11). Three out of 29 patients had developed doubling of serum creatinine in the early acute ABMR group compared to 12 out of 40 patients in the late acute ABMR group, which was statistically significant (P < 0.05). Three patients in each group had died, which was not statistically significant. Kaplan-Meier survival graph comparing acute vs late ABMR group for graft survival and doubling of serum creatinine is depicted in Figures 1 and 2 respectively.

- Kaplan–Meier survival graph for comparing the graft survival after ABMR episode. LOG RANK: 0.272
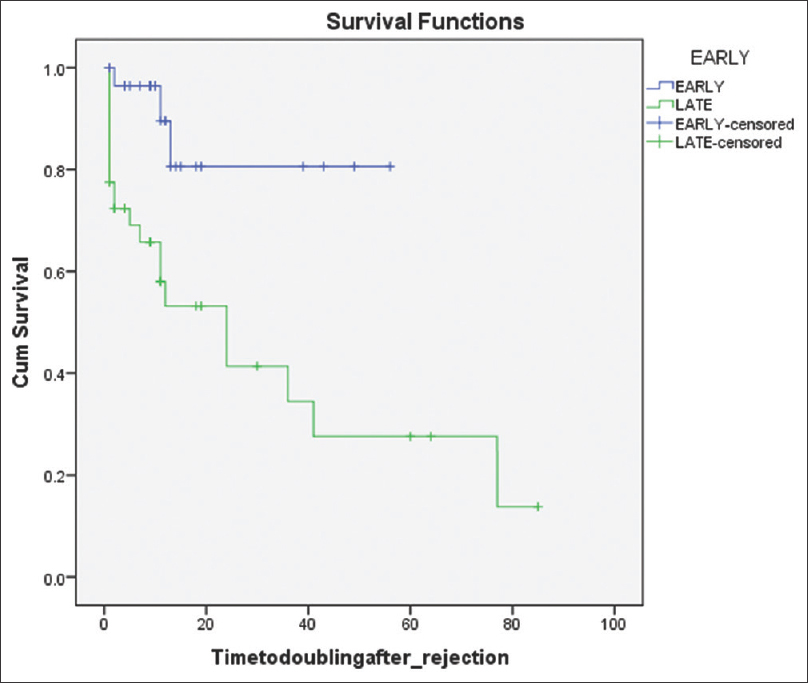
- Kaplan–Meier survival graph for comparing the doubling of S.creatinine after ABMR episode. LOG RANK: 0.002
Infectious complications were not different between the two groups. The frequencies of CMV disease and fungal infections were not statistically different between the two groups.
Discussion
The major finding of this study was that late ABMR (>3 months of transplantation) was associated with an increased risk of doubling of serum creatinine and also had poor response to plasmapheresis compared to early acute ABMR. Even among the 28 patients in late acute ABMR who responded to plasmapheresis initially, 10 patients (35.7%) had doubling of serum creatinine during the follow-up period. This clearly establishes that late acute ABMR has different pathophysiology compared to early acute ABMR as mentioned by previous studies.[5] The continuous production and presence of DSA in late acute ABMR may be the reason for the doubling of serum creatinine even after the initial response to plasma exchange. However, in our study, the presence of Denovo DSA was not documented in all late acute ABMR patients, which is one of the limitations of this study.
Suboptimal immunosuppression and nonadherence were documented in most of the patients in the late acute ABMR group. Thus, increased attention to compliance with the drug regime is important in preventing late acute ABMR. Poor adherence to immunosuppression as a cause for late ABMR has been documented in various studies[8,11-13] In previous studies, young age and complex medication schedules were associated with nonadherence, which was not seen in our study.[14]
Previous studies by Dorje et al.[8] and Walsh et al.[5] found that late ABMR had been associated with poor graft survival. In this study, we could not demonstrate any significant difference in graft or patient survival between the two groups. This may be because of the short follow-up time in the study group. However, it was found that the late acute ABMR group had an increased possibility of doubling of serum creatinine than the early acute ABMR group. Large registry studies by Opelz et al.[15] and Lentine et al.[16] found that late rejection regardless of the type of rejection was associated with inferior graft survival. Delay in diagnosis may be one of the factors for nonresponse to plasma exchange in the late acute ABMR group. Most of the rejections in early acute ABMR happened during the hospital stay. This may be one of the reasons for the response to plasmapheresis in which the therapy is instituted without time delay.
In our study, biopsies that had chronic changes such as transplant glomerulopathy had been excluded. In previous studies,[8] it was mentioned that chronic changes in late ABMR were one of the reasons for poor response and inferior graft survival. By excluding the biopsies with chronic changes, in our study, we demonstrated that late acute ABMR had increased risk for doubling of serum creatinine even without chronic changes on biopsy by light microscopy. This also explains the different pathophysiologic mechanisms of late acute ABMR as described by the previous studies.[10] However, in our study, electron microscopy of biopsy specimens was not done, which is one of the limitations of this study. In previous studies,[8] late acute ABMR was associated with an increased number of combined rejections than early acute ABMR patients. This finding was not present in our study even though nonadherence was more common in the late acute ABMR group.
One patient in the early acute ABMR group and three patients in the late acute ABMR group received rituximab injection in doses of 375 mg/m2 in addition to plasmapheresis and IVG. Among the four patients, two patients had doubling of serum creatinine and one patient developed allograft failure during the follow-up period. Rituximab was used in these patients due to poor response to plasmapheresis. These patients also received extended sessions of plasmapheresis. The response rate in rituximab-treated patients was poor. This is similar to the case series done by Surendra et al.[17] in refractory ABMR patients treated with rituximab.
Bortezomib was given to one patient who was refractory to plasmapheresis, IVIG, and rituximab. Later, that patient developed allograft failure and died due to pneumonia. As this drug was used in only one patient, we could not derive any conclusion based on this finding. However, in a study done by Waiser et al.,[18] addition of bortezomib to rituximab, plasmapheresis and IVIG was not found to be beneficial in improving graft function. Instead, these patients developed increased adverse events.
Infectious complications such as fungal infection and CMV disease were not different between the early and late acute ABMR groups in our study even though more patients in the late acute ABMR group received rituximab. This is in contrast to the study done by Dorje et al.[8] where early acute ABMR group patients had an increased risk of CMV infection. As late acute ABMR may be refractory compared to early acute ABMR, it may need further additional therapies such as rituximab and bortezomib in addition to plasmapheresis IVIG, which requires further evaluation in further randomized trials.
Late acute ABMR is fundamentally different from early acute ABMR for several reasons.[7] In this study, we have documented that most of the late acute ABMR patients had inappropriate subnormal CNI levels due to noncompliance or reduced dosage. These patients also have less frequent visits to clinic compared to early acute ABMR patients, which increases the likelihood of nonadherence, thereby precipitating ABMR. In our study, the majority of ABMR episodes were DSA negative and would qualify for the label of suspicious ABMR diagnosis as per the updated Banff classification. Antibodies to non-HLA antigens had not been evaluated in our study population, which is one of the limitations of this study. A probabilistic assessment approach for making the diagnosis of ABMR is more clinically useful in patients with typical histological lesions of ABMR[2]
Conclusion
The late acute ABMR group had a poor response to plasmapheresis and an increased risk of doubling of serum creatinine compared to the early acute ABMR group. There was also a tendency toward increased graft loss in late acute ABMR patients. Some of the patients with late acute ABMR showed initial partial response to plasmapheresis, which was better and more sustained in early ABMR. Infectious complications were not different between the two groups. Several patients with late acute ABMR had noncompliance issues in terms of immunosuppressive drugs and may need different strategies of treatment compared to early acute ABMR patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- OPTN/SRTR 2012 annual data report: Kidney. Am J Transplant. 2014;14((Suppl-1)):11-44.
- [Google Scholar]
- Banff 2013 meeting report:Inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272-83.
- [Google Scholar]
- Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant. 2015;15:489-98.
- [Google Scholar]
- Plasmapheresis and intravenous immunoglobulin in early antibody-mediated rejection of the renal allograft:A-single-center experience. Ther Apher Dial. 2009;13:108-12.
- [Google Scholar]
- Early and late acute antibody-mediated rejection differ immunologically and in response to proteasome inhibition. Transplantation. 2011;91:1218-26.
- [Google Scholar]
- One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation:RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation. 2016;100:391-9.
- [Google Scholar]
- Banff 2011 Meeting report:New concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563-70.
- [Google Scholar]
- Early versus late acute antibody-mediated rejection in renal transplant recipients. Transplantation. 2013;96:79-84.
- [Google Scholar]
- Late and early C4d-positive acute rejection:Different clinico-histopathological subentities in renal transplantation. Kidney Int. 2006;70:377-83.
- [Google Scholar]
- Early and late humoral rejection:A-clinicopathologic entity in two times. Transplant Proc. 2008;40:3229-36.
- [Google Scholar]
- Prospective study on late consequences of subclinical non-compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant. 2004;4:1509-13.
- [Google Scholar]
- Frequency and impact of nonadherence to immunosuppressants after renal transplantation:A-systematic review. Transplantation. 2004;77:769-76.
- [Google Scholar]
- Adherence with immunosuppressive treatment after transplantation:Results from the French trial PREDICT. Clin Transplant. 2012;26:E293-9.
- [Google Scholar]
- Factors related to immunosuppressant medication adherence in renal transplant recipients. Clin Transplant. 2012;26:706-13.
- [Google Scholar]
- Influence of time of rejection on long--term graft survival in renal transplantation. Transplantation. 2008;85:661-6.
- [Google Scholar]
- The implications of acute rejection for allograft survival in contemporary U.S. kidney transplantation. Transplantation. 2012;94:369-76.
- [Google Scholar]
- Rituximab in the treatment of refractory late acute antibody-mediated rejection:Our initial experience. Indian J Nephrol. 2016;26:317-21.
- [Google Scholar]
- Rituximab in combination with bortezomib, plasmapheresis, and high-dose IVIG to treat antibody-mediated renal allograft rejection. Transplant Direct. 2016;2:e91. doi:10.1097/TXD.0000000000000604
- [Google Scholar]







