Translate this page into:
Comparison of azathioprine with mycophenolate mofetil in a living donor kidney transplant programme
Address for correspondence: Dr. Vijay Kher, Department of Nephrology, Medanta Kidney and Urology Institute, Medanta- The Medicity, Sector 38, Gurgaon- 122 001, Haryana, India. E-mail: vijay.kher@medanta.org
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
There are conflicting data regarding the comparative efficacy of mycophenolate mofetil (MMF) versus azathioprine (AZA) as maintenance immunosuppressive agent in kidney transplantation. The data are even less in combination with tacrolimus (TAC) in living donor kidney transplantation. A total of 205 living donor kidney transplants, on TAC-based triple drug immunosuppression were included in the study. A total of 113 patients received AZA and rest 92 were on MMF based protocol. TAC levels were monitored and graft biopsy was done whenever rejection was suspected. The outcomes were evaluated in terms acute rejection (AR) episodes at 1 year, infections, renal function, graft loss, and death between two groups. The study group comprised 163 males (79.5%) and 42 (20.5%) females. The mean age of patients was 42.4±11.8 years in the AZA group and 39.4 ±13.4 in the MMF group (P=0.09). The mean duration of follow-up was 491.7±240.7 and 478.8±334.4 days respectively in the AZA and MMF groups (P=0.75). Thirty-seven of 92 (40.2%) patients in the MMF group and 70/113 (61.9%) patients in the AZA group received IL-2 RAb induction (P=0.002). 32 patients (15.6 %) developed AR within a year. The incidence of AR was similar in patients who received MMF (12/92, 13%) and those who received AZA (20/113, 17.5%), (P=0.36). There was no difference in the incidence of AR in the subgroup of patients who received IL-2 RAb compared to those who did not receive induction in the two groups (5/37 vs. 7/55 in the MMF group and 10/70 vs. 10/43 in the AZA group, P=0.72). The incidence of infections was similar in the two groups (19/92, 20.6% vs. 25/113, 22.1%, P=0.79). Three patients developed CMV disease, of which two were in the MMF group. Graft loss occurred in 7/205 (3.4%) and death in 8/205 (3.9%) patients. Six of eight patients who died had functioning grafts. The rate of graft loss (3/92 vs. 4/113, P=0.97) and death (5/92 vs. 3/113, P=0.27) was similar in two groups. The overall patient survival was 94.5% and death censored graft survival was 97.4%. Cost comparison suggests AZA to be 6-10 times cheaper than MMF. This study suggests that, in tacrolimus-based immunosuppression, azathioprine may be as good as MMF as maintenance immunosuppressive drug in living donor kidney transplantation. It is also a more cost-effective immunosuppression.
Keywords
Azathioprine
kidney transplant
living donor
mycophenolate mofetil
Introduction
Azathioprine (AZA) is an inhibitor of purine synthesis and has been used as an immunosuppressant since 1960s.[1] Mycophenolate mofetil (MMF) is a more selective inhibitor of purine, as it causes reversible inhibition of inositol monophosphate dehydrogenase, in particular the type II enzyme which is expressed predominantly in activated lymphocytes.[2] MMF replaced AZA as predominant antimetabolite agent in kidney transplantation after publication of three pivotal multicenter randomized controlled trials (RCTs), in the early 1990s. Pooled efficacy analysis of these three trials demonstrated a significant reduction in acute rejection rates with use of MMF compared to AZA or placebo along with cyclosporine A (CsA) and steroids.[3] Additional studies later on also suggested a reduced incidence of both early and late acute rejection episodes among patients treated with MMF.[45]
However, recently with the publication of MYSS and MYSS follow-up trials,[67] there has been a resurgence of interest in the use of azathioprine as maintenance immunosupression. The MYSS trialsrandomized cadaveric renal transplant recipients de novo to MMF or AZA along with CsA microemulsion (ME) and steroids withdrawal protocol. No difference was noted in acute rejection rates and graft survival in two groups.
Introduction of tacrolimus (TAC) in the late 1990s in kidney transplant led to further reduction in acute rejection rates, compared to the CsA-based regimen,[89] In fact TAC and MMF have become predominant immunosuppressive regimen in kidney transplant recipients in most of the centers nowadays.[10] But there are very few studies that have compared AZA with MMF as maintenance immunosuppression in TAC-based protocols and the results are variable.
In a study by Gonwa et al,[11] who conducted a randomized trial in recipients of first cadaveric kidney allograft, comparing TAC+MMF, TAC+AZA, and CsA+MMF, acute rejections, graft and patient survival at 1, 2, and 3 years were similar in all three groups. In a recent meta-analysis by Morris et al,[13] there was a significant reduction in the incidence of AR in the MMF group compared to AZA, and graft loss was also more in the AZA group.[12] But the effect was least significant with tacrolimus compared to cyclosporine. In a registry analysis there was no difference in the graft and patient survival between MMF versus AZA, when combined with CNIs; however the incidence of acute rejection was higher in the AZA group. However most of these studies are done in recipients of cadaveric donors, who have inferior short- and long-term graft survival compared with recipients of living donors.[14]
In India, in the absence of any governmental payment or subsidy for the care of CKD or kidney transplant, patients have to pay for their treatment. The cost of treatment is an important determinant of the type of immunosuppressive combination a patient receives. Our group continue to use azathioprine in a significant number of patients. We evaluated the outcomes in our living donor first kidney transplant recipients, comparing AZA versus MMF in combination with tacrolimus and steroids. The outcome measured was incidence of acute rejection at 1 year, infections, graft, and patient survival.
Materials and Methods
A total of 266 living donor kidney transplantations were done at our hospital in the national capital region (NCR) of Delhi, between May 2006 and April 2009. Of these 53 patients who were on cyclosporine-based regimen (n=28) or steroid-free protocol (n=25) were excluded from the study. Eight patients, who had undergone second transplantation, were also excluded from the analysis (7 in the MMF group and 1 in the AZA group). The exclusions were done to make the groups more uniform. We do not routinely do panel-reactive antibodies (PRA) in our first transplant recipient. The crossmatch done was standard CDC lymphocytotoxic crossmatch. The study group comprised 205 consecutive kidney transplant patients. The maintenance immunosuppressive protocol consisted of TAC, MMF or AZA, and steroids. Immunosuppressive protocols and induction with IL-2 receptor antibody (IL-2 RAb) were discussed with the patient and the family and after due diligence, and ability to afford long-term sustenance of therapy; patients received IL-2 RAb/no IL-2 RAb, and MMF or azathioprine. All patients received perioperative methylprednisolone 500 mg iv followed by tablet prednisone 40 mg once a day from the next day, tapered to 20 mg on day 10. After that, it was gradually tapered to 7.5 mg at the end of 3 months. Most of the patients were receiving prednisolone 5 mg once a day after 6 months, if their course was uneventful. Tacrolimus was started on day minus 1 of transplant at a dose of 0.1 mg/kg/wt in two divided doses and TAC whole blood trough levels were done on day 4 and 8 during hospitalization, and after that at least once a month or whenever required. TAC levels were maintained between 8-12 ng/ml in first 3 months, 6-8 ng/ml next three months and 4-6 ng/ml thereafter. MMF was started day minus 1 of transplant in a dose of 1000 mg twice a day and azathioprine in a dose of 2 mg/kg/wt. once a day. The dose of MMF was reduced to 1.5 gm/day after 1 month if levels of TAC were adequate and subsequently to 500 mg twice a day if the course was uneventful. The dose of azathioprine was reduced to 1.5 mg/kg after few days. These patients were followed up twice weekly in the first month, once a week for the next 2 months, once in 2 weeks for the next 3 months and once a month thereafter. At each visit complete blood counts, BUN, and serum creatinine were done and other investigations were done as per protocol at our centre. Kidney biopsy was done in the event of graft dysfunction except when a patient did not give consent or was unsuitable for a biopsy. In the event of biopsy-proven or clinical rejection, the patients were treated initially with iv methylprednisolone 250-500 mg infusion for 3-5 days. If the response was not satisfactory, then he/she was labeled as steroid resistant and was treated with thymoglobulin (rabbit ATG, Genzyme) at a dose of 1.5 mg/kg/dose between 3 and 7 doses. ATG was always given after a kidney biopsy. A patient was labeled as having refractory rejection, if he did not respond to pulse steroids and ATG. The primary outcome was incidence of acute rejection at 1 year. Secondary outcomes studied were incidence of infections, new onset diabetes after transplant, last serum creatinine, graft and patient survival.
Statistical analysis
Univariate analysis using Student's t-test for continuous variables and chi-square test and Fisher exact test for categorical variables were performed. Graft and patient survival were analyzed using univariate Kaplan-Meier plots and log rank tests. Graft survival was defined as time taken from transplantation to failure, censoring for death with a functioning graft. All analysis was performed using SPSS software (version 11). Statistical significance in all analysis was assumed if the P value was less than 0.05. A post hoc analysis was done at the end of the study.
Results
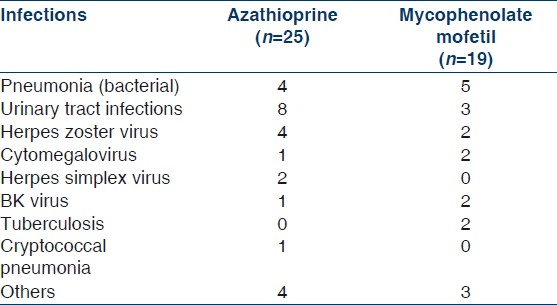
The demographics and clinical variables of patients are given in Table 1. Out of these 113 patients received AZA and 92 received MMF. In addition 107 (52.1%) patients received IL-2 RAb. The mean age was similar in the two groups (42 vs. 39 years). There was no difference in other parameters also: gender, diabetics, HLA matches and mean duration of follow-up in two groups. Of the 205 patients, 32 (15.6%) had acute rejection episodes, out of which 29 (14.1%) had biopsy-proven acute rejections [Table 2]. When we compared the two groups, the incidence of acute rejection was 13% (12/92) in the MMF group and 17.6% (20/113) in the AZA group, which was not statistically significant (P=0.36). Two patients in the MMF group and one patient in the AZA group had clinical rejection. The incidence of biopsy-proven acute rejection was also similar in both groups (10.8% in the MMF group vs. 16.6% in the AZA group,). Although a significantly greater number of patients in the AZA group received IL-2 RAb (70/113) compared to the MMF group (37/92, P=0.002), on subgroup analysis, the use of IL-2 RAb was not associated with reduced incidence of acute rejection in any of the two groups (5/37 vs. 7/55 in the MMF group and 10/70 vs. 10/44 in the AZA group, P=0.72) [Table 3]. Three patients in the AZA group and two in the MMF group received thymoglobulin (rabbit ATG- genzyme) for steroid resistant rejection, and one patient lost his graft due to refractory rejection in the AZA group.

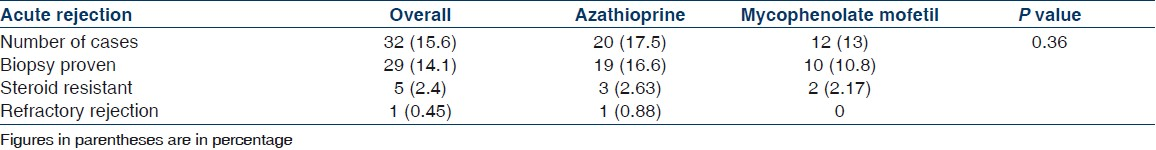

The incidence of infections was also not different between two protocols [Tables 4 and 5]. In the MMF group 19/92 patients (20.6%) had infections compared to 25/113 (22.1%) in the AZA group (P=0.79). One patient in each group died of pneumonia. The incidence of CMV infection was low overall, 2/92 (2.17%) in the MMF group and 1/113 (0.88%) in the AZA group. Two patients in the MMF group had BKV infection; both of them lost their graft, one of these patients also had coexistent CMV infection. One patient in the AZA group developed cryptococcus pneumonia, which improved after treatment. The incidence of new onset diabetes after transplant (NODAT) was also similar in the two groups (9 in each group, P=0.64). Last follow-up mean serum creatinine was 1.52±1.21 in the AZA group and 1.64±1.33 in the MMF group, which was not different statistically (P=0.89) [Table 5]. There was no malignancy in the AZA or MMF group till last follow-up. There was no drug withdrawal related to hematological adverse events. Dose modifications were done in some patients, which corrected minor adverse events.

Four (3.5%) patients in the AZA group and three (3.2%) patients in the MMF group lost their grafts. The causes of graft loss in the MMF group were drug default in one case, BKV infection in one patient, and combined CMV/BKV infection in one patient. In the AZA group, one patient lost graft after resistant acute rejection, one patient had recurrence of FSGS, one patient had graft pyelonephritis, and one patient was drug defaulter. A total of eight patients died during follow-up, three in the AZA group (2.6%) and five in the MMF group (5.4%). The cause of death in the AZA group was sepsis in all the three patients. In the MMF group three patients died of sepsis, one patient died of acute myocardial infection, and one patient missed dialysis after graft loss and died at home. Graft survival and patient survival were similar in the two groups. Six of eight patients died with functioning grafts. In the AZA group, the death-censored graft survival was 96.5% and in the MMF group, 96.7% (log rank, P=0.97, Figure 1). Patient survival was 94.5% in the MMF group and 97.4% in the AZA group (log rank= 0.27, Figure 2).
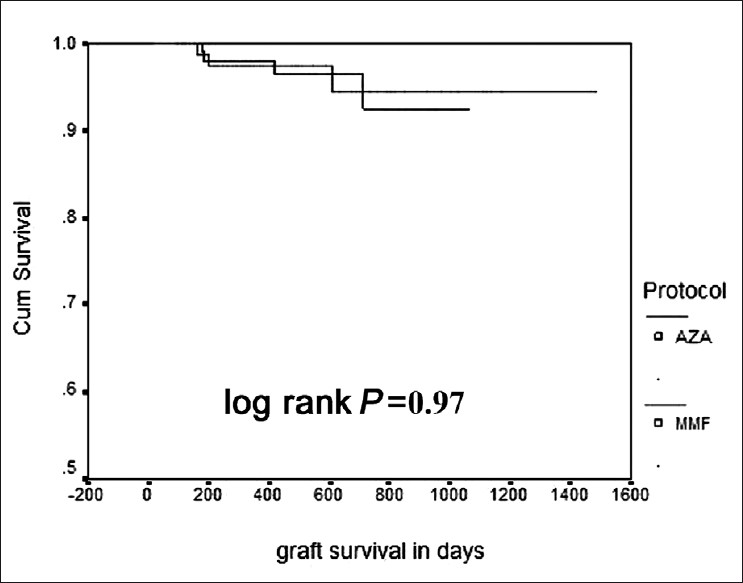
- Kaplan-Meier curve for graft survival in the two groups (Upper line MMF, lower line AZA)
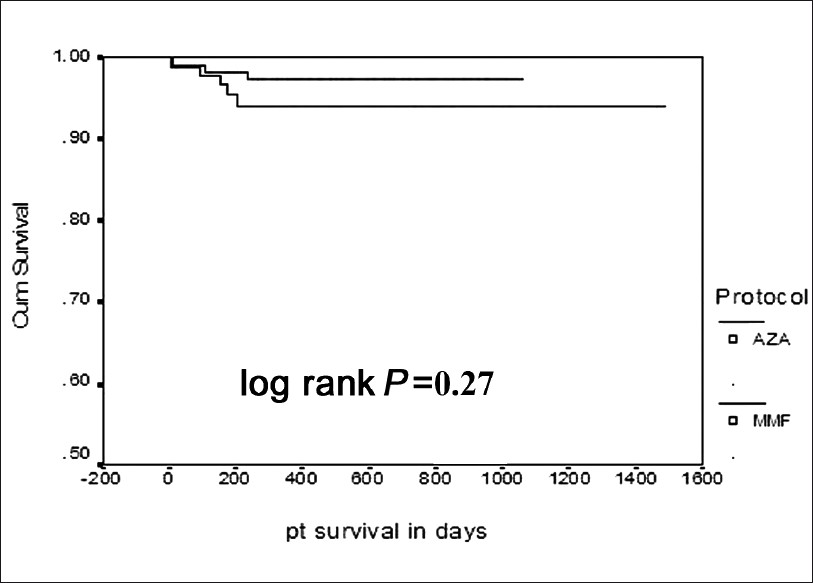
- Kaplan-Meier curve for patient survival in the two groups (Upper line AZA, lower line MMF)
The costs of AZA and MMF
The average daily dose of MMF was 1500 mg for the first 3 months and 1000 mg after that and the dose of AZA was 100 mg/day in most of our patients. The cost of MMF (Cellcept- Roche) is Rs 60/tab, of 500 mg, i.e., Rs 5500/month for first 3 months and thereafter –Rs. 3600/month. The cost of AZA (Azoran- RPG) is Rs 10/month/tab of 50 mg. The monthly cost of therapy is approximately US 500- 600/month which is approximately 6-10 times less than MMF. Even the generic MMF is 5-8 times more expensive than azathioprine.
Discussion
The present study shows no difference in outcomes including BPAR, renal function, 1-year patient and death-censored graft survival comparing the MMF and AZA groups in patients on triple drug immunosuppression comprising tacrolimus and steroids in our living donor kidney transplant program. This finding is important, as TAC+AZA+ steroid is the most cost-effective maintenance immunosuppressive regimen.
In initial trials comparing AZA or placebo versus MMF, the older cyclosporine formulation was used, which did not have reliable absorption.[3–5] In pooled efficacy analysis of three pivotal randomized trials there was no significant difference in graft or patient survival between MMF and AZA or placebo arms.[3]
When MMF was compared to AZA along with cyclosporine microemulsion in a prospective randomized MYSS study, there was no difference in the rates of acute rejection at 21 months between two groups. The adverse effect profile was also similar in AZA and MMF arm.[4] The authors extended the study to see the long-term effect of two drugs on GFR, proteinuria, late rejections, and mortality. They did not find any benefit of MMF over AZA in 5-year outcomes also. The authors recommended that AZA should be used in place of MMF in kidney transplant, because of its cost-effectiveness (15 times more cost effective) and no additional benefits with MMF.[5]. Similarly in a paired kidney analysis by Shah, et al, there was no difference in graft or patient outcomes in the MMF and AZA groups, when used in triple immunosuppression comprising CNIs (TAC or CsA ME) and steroids. On the contrary MMF therapy was associated with increased acute rejection rates. The authors explained this increased rejection rate in the MMF group possibly due to selection of high-risk patients in this group.[15] Miller, et al.[16] reported results of a randomized prospective trial with three arms including AZA, low, and high doses of MMF in combination with tacrolimus and showed lower acute rejection rates with high but not low doses of MMF compared to AZA and otherwise similar rates of adverse events. These trials suggest that the beneficial effects of MMF compared to AZA may be lost when patients are treated with cyclosporine microemulsion or tacrolimus. In addition, a randomized trial comparing Neoral and MMF with tacrolimus and MMF or AZA showed similar overall biopsy-proven acute rejection rates and graft survival in all groups at 1 and 3 years.[11]
Interleukin 2 receptor antibodies have been demonstrated to reduce the incidence of acute rejection.[17] IL-2 RAbs reduced acute rejections in our patient population in the cyclosporine, azathioprine, and prednisolone groups by 50 %.[18] However, with the use of tacrolimus-based immunosuppression, IL-2 RAb had no impact on incidence of acute rejection in this study. Although the IL-2 RAb use was significantly more in the AZA group compared to MMF (P=0.002), but there was no difference in the incidence of acute rejection in the AZA or MMF group with or without IL-2 RAb use. This may be explained by first transplant- recipients of living donors as compared to cadaveric donors or subsequent transplant, who are at higher immunological risks. Similar results have been published by Lim et al.[19] in their registry analysis, in which they have shown that there was no effect of IL-2Ra induction on incidence of AR in low immunological risk recipients as well as intermediate immunological risk recipients on tacrolimus-based regimen.
A significant finding in demographics is that about 80% of our recipients were male and 70% of donors were female in both the groups. This is due to socioeconomic factors in our population, because the male is usually the earning member of a family. All other baseline parameters, e.g., HLA matches, mean duration of follow-up, and basic disease, were similar in both groups. The incidence of adverse events like NODAT and infections including CMV and BKV were similar in the two groups. The overall incidence of CMV was low in our transplant patients (3/205). This may be explained by overall low immunosuppression and rapid tapering of steroids in our patients.
Recently two large studies have been published comparing MMF versus AZA in combination with calcineurin inhibitors. In a meta-analysis of 19 RCT by Morris, et al there was a significant decrease in the acute rejection rates with MMF compared to AZA, and the graft loss was also lower in the MMF group. But the main problem of this analysis was heterogeneity of included studies and a possible publication bias toward inclusion of studies with positive outcomes as discussed by the author himself. Secondly the difference in acute rejection was seen most significant with cyclosporine and less with cyclosporine microemulsion. The difference in acute rejection rate was least significant with tacrolimus. There was no difference in patient survival and adverse events between the two groups.[12]
In a retrospective analysis of large registry data of the international collaborative transplant study (CTS) by Opelz, et al.[13] between 1998- 2007, which included more than 50,000 patients, there was no difference in graft and patient survival between the AZA and MMF groups, but there was significantly higher rates of acute rejections and significantly lower risk of hospitalization in the AZA group compared to the MMF. This registry analysis concludes that there is no long-term graft survival benefit of MMF over AZA.[13]
Recently published K-DIGO guidelines for kidney transplant recipients suggest that mycophenolate mofetil be the first-line antiproliferative agent (2B). However, it also mentions that, because of the substantially increased cost of MMF compared with azathioprine, there is no clear cut net benefit, but a decision based upon trade-offs is required.[20]
Current guidelines for the use of immunosuppressants in the United Kingdom states that MMF should only be used where there is proven intolerance to CNIs or in situations where there is a high risk of nephrotoxicity requiring CNI minimization or reduction.[21] This recommendation is based on the pooled analysis of seven RCTs which demonstrates a reduction in acute rejection but no improvement in graft survival with MMF, along with significantly higher cost of MMF compared to AZA. We also demonstrated that MMF is 6-10 times more costly compared to AZA in our patients and it does not give any additional benefit in terms of acute rejections, adverse events, and graft survival.
Our study has certain limitations: it is not a randomized study, and thus cannot exclude selection bias. Economic factors may have influenced selection of poorer patients to the AZA group; however despite this, AZA group did not have inferior outcomes and therefore this does not go against the validity of the study. Secondly our study had a smaller number of patients and post hoc analysis revealed that this study was underpowered to detect the difference in acute rejection in the AZA and MMF groups. Post hoc analysis also revealed that the study was not powered enough to detect the difference in acute rejection rates in the IL-2 subgroup; therefore it is difficult to draw any conclusion regarding its use or nonuse. A larger randomized and adequately powered study is required to address this issue. Another limitation of this study was a shorter follow-up and it is possible that results might change in the long term.
To conclude, our study suggests that in our living-donor first kidney transplant recipients, there is no difference in the rates of acute rejection at 1 year, adverse events, graft and patient survival between the AZA and MMF groups in tacrolimus+ steroid-based immunosuppression.
Source of Support: Nil
Conflict of Interest: None.
References
- Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation. 1997;63:39-47.
- [Google Scholar]
- Mycophenolate mofetil versus azathioprine therapy is associated with a significant protection against long-term renal allograft function deterioration. Transplantation. 2003;75:1341-6.
- [Google Scholar]
- Mycophenolate mofetil vs azathioprine in a large population of elderly renal transplant patients. Nephrol Dial Transplant. 2004;19:2864-9.
- [Google Scholar]
- Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): a randomized trial. Lancet. 2004;364:503-12.
- [Google Scholar]
- Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: the MYSS follow-up randomized, controlled clinical trial. J Am Soc Nephrol. 2007;18:1973-85.
- [Google Scholar]
- A comparison of tacrolimus (FK506) and cyclosporine for immunosupression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation. 1997;63:977-83.
- [Google Scholar]
- Tacrolimus versus ciclosporin as primary immunosupression for kidney transplant recipients: meta- analysis and meta-regression analysis of randomized trial data. BMJ. 2005;331:810.
- [Google Scholar]
- Immunosuppression: evolution in practice and trends, 1994-2004. Am J Transplant. 2006;6:1111-31.
- [Google Scholar]
- Randomized trial of tacrolimus + mycophenolate mofetil or azathioprine versus cyclosporine + mycophenolate mofetil after cadaveric kidney transplantation: results at three years. Transplantation. 2003;75:2048-53.
- [Google Scholar]
- Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: A systematic review. Transplantation. 2009;87:785-94.
- [Google Scholar]
- Collaborative Transplant Study.Influence of immunosuppressive regimens on graft survival and secondary outcomes after kidney transplantation. Transplantation. 2009;87:795-802.
- [Google Scholar]
- Long-term graft outcome with mycophenolate mofetil and azathioprine: A paired kidney analysis. Transplantation. 2006;82:1634-9.
- [Google Scholar]
- Safety and efficacy of tacrolimus in combination with mycophenolate mofetil (MMF) in cadaveric renal transplant recipients. FK506/MMF Dose-Ranging Kidney Transplant Study Group. Transplantation. 2000;69:875-80.
- [Google Scholar]
- Interleukin-2 receptor antibody reduces rejection rates and graft loss in live-donor kidney transplant recipients. Transplantation. 2009;88:1208-13.
- [Google Scholar]
- Basiliximab in renal transplantation: Experience from single centre. Transplant Proc. 2004;36:621-2.
- [Google Scholar]
- Interleukin-2 receptor antibody does not reduce rejection risk in low immunological risk or tacrolimus-treated intermediate immunological risk renal transplant recipients. Nephrology (Carlton). 2010;15:368-76.
- [Google Scholar]
- Kidney disease: Improving global outcomes. Transplant work group. KDIGO Clinical Practice Guidelines for the Care of Kidney Transplant Recipients. Am J Transplant. 2009;9(Suppl-3):S10-S13.
- [Google Scholar]
- National Institute for clinical excellence. Technology appraisal 85: Immunosuppressive therapy for renal transplantation in adults. Available from: http://www. nice.org.uk/TA085guidance
- [Google Scholar]







