Translate this page into:
Correlation of AKI with Risk Factors, Ventilatory Support, Renal Replacement Therapy in a Cohort of COVID-19 Patients
Address for correspondence: Prof. Georgi Abraham, MGM Healthcare, No 54, Nelson Manickam Road, Aminjikarai, Chennai - 600 029, Tamil Nadu, India. E-mail: Abraham_georgi@yahoo.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
There is a scarcity of information on the incidence and outcomes of acute kidney injury (AKI) in COVID-19 patients in India. Therefore, we analyzed the correlation of AKI risk factors, ventilatory support, and renal replacement therapy and compared the outcomes of first and second COVID-19 waves in this tertiary care center.
Methods:
We retrospectively analyzed the patients' medical records with a positive RT-PCR for COVID-19 between July 2020 and May 2021. We looked at the clinical outcomes of the first and second COVID-19 waves and documented the frequency, risk factors for AKI, and the relationship between AKI and in-hospital mortality. Univariate and multivariate binomial logistic regression yielded odds ratios for the risk variables of AKI. Risk differences and age-adjusted odds ratios, as well as 99.5% confidence intervals, were used to compare COVID-19 outcomes between the first and second waves.
Results:
Of the 1260 hospitalized patients with COVID-19, 86 (6.8%) presented with AKI and 8 (0.7%) patients required dialysis. The most common comorbidity was diabetes mellitus (55.2%), hypertension (42.1%), hypothyroidism (11.3%), and coronary artery disease (8.1%). A total of 229 (18.17%) patients were admitted to ICU, 574 (45.5%) received ventilation, and 26 (2.0%) required mechanical ventilation.The incidence of in-hospital death in the patients with AKI as per the stage from 1 to 3 was 9 (15.8%), 7 (35%), and 5 (55.6%), respectively.Compared to the first wave, the second wave cohort had a lower risk of AKI (adj OR: 0.426; CI: 0.232–0.782) and mortality (adj OR: 0.252; CI: 0.090–0.707).
Conclusions:
In our study, AKI prevalence was 6.8%, the need for ventilation was 45.5%, ECMO 0.2%, and the mortality rate 2.9%. Second wave of COVID-19 exhibits improved clinical outcomes compared to the first wave.
Keywords
Acute kidney injury
comorbidities
COVID-19
Introduction
A series of acute respiratory illness cases of unknown origin were reported in Wuhan, China, in December 2019.[12] As of July 2021, 196 million cases have been reported, with 4.2 million deaths. COVID-19 infection had a significant impact on healthcare in India in terms of morbidity and mortality. COVID-19 is most known for its pulmonary symptoms, although acute kidney injury (AKI) is increasingly recognized as a frequent consequence of the condition. The factors that may play a role in COVID-19-related AKI include endothelial injury,[34] microvascular thrombi, local inflammation,[5] and immune cell infiltration of the kidney.[6] COVID -19 has also been found to interact with the human angiotensin-converting enzyme II molecule.[7] Although the reported frequency of AKI among COVID-19-infected hospitalized patients varies substantially, the various studies that looked at COVID-19-related incidence of kidney involvement thus far reported a wide range of incidence, between 0.5% and 43% in hospitalized patients[8] and >50% in patients admitted to ICU. However, the incidence of kidney disease is low in patients unless they had[910] preexisting renal disease, hypertension, cardiovascular disease, diabetes mellitus, older age, or severe hypoxemia.
India, the world's second-most populated country, has the second highest COVID-19 burden. However, information on the occurrence and outcomes of AKI in COVID-19 on larger studies in India is limited.[111213] This single-center, retrospective study, aimed to report the risk factors, incidence, outcomes, and mortality among patients with acute kidney injury in COVID-19. Between the first and second waves of the pandemic, we studied demographic factors and clinical outcomes of those infected with COVID-19.
Materials and Methods
Study cohort
This retrospective study was conducted in MGM Healthcare (Chennai, India). We manually extracted the data of 1867 hospitalized patients who tested positive for COVID-19 by real-time reverse transcriptase-polymerase chain reaction between July 2020 and May 2021. We included patients who were aged at least 10 years or above. A total of 1260 patients who had their serum creatinine levels measured on admission were included in the study [Figure 1]. We excluded 1) patients with end-stage kidney disease (ESKD), 2) those with missing admission serum creatinine, and 3) those for whom hospitalization lasted <24 h. By the time of this analysis, all patients had either died or were discharged from the hospital. The treatment consisted of IV dexamethasone/ methylprednisolone, remdesivir, azithromycin, anti-fungal agents, and other supportive nutritional therapy as per the physicians, nephrologists, and ICU staff discretion. The Institutional Human Ethics Committee of MGMC & RI approved the study. (MGMCRI/IRC/38/2021/04/IHEC/37, dated: 21/06/2021)

- Flowchart of the study population of COVID-19 patients
Data collection
The clinical symptoms, co-morbidities, demographic parameters, radiological investigations, and outcomes were manually extracted from the medical records department for analysis. In addition, we collected details such as ICU stay, mechanical ventilation, and baseline laboratory test results with 48 h of hospital admission. The laboratory data consisted of WBC count, kidney function tests, liver function tests, D-dimer, C-reactive protein, and ferritin. In addition, urine analysis, lactate dehydrogenase, and Il-6 were selectively tested according to the patient's condition. The data were reviewed by three independent nephrologists and two internal medicine physicians.
Definitions of outcomes
AKI, the primary endpoint was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria as follows: stage 1, as an increase in serum creatinine (Scr) level by 0.3 mg/dL with 48 h or 1.5–1.9 times increase in Scr level from baseline within 7 days; stage 2, as 2–2.29 times increase in Scr levels within 7 days; and stage 3, as ≥3 times increase in Scr level within 7 days or initiation of dialysis.[14] Patients were stratified according to the most severe stage of AKI. As we had no access to the past medical records, the stage of AKI was determined if the serum creatinine value was 0.3 mg/dL more than the upper limit (1.17 mg/dL). The estimated glomerular filtration rate (eGFR) was calculated using CKD epidemiology collaboration (CKD-EPI) creatinine equation.[15] On urine analysis, proteinuria was classified as ≥1+ proteinuria (trace was counted as no proteinuria). Hematuria was defined as >4–6 red blood cells per high-power field on urine analysis. Leukocyturia was defined as >4–6 white blood cells per high-power field. The severity of COVID-19 infection was defined on standard line.[16]
Statistical analysis
We compared the 1260 patients with confirmed COVID-19 with no AKI to those who developed different AKI stages during the hospital stay. Various categorical variables are stated in percentages. Moreover, the frequency count and the median and interquartile range are reported for the continuous skewed variables in the first and third quartile.
The differences in baseline characteristics between patients with different stages of AKI and without AKI were not analyzed by the usual Chi-square test for association among the two categorical variables as the expected count of many cells was less than 5 in the contingency tables of various nominal categories. This led to the fact that the regular test of association, the chi-square test, became inapplicable. Thus, the most appropriate test in this situation for the 2 × 2 contingency table is Fisher's exact test, and for the more than two order contingency table is the Freeman–Halton extension of Fisher's exact test. These two tests of association were used to compute the association among the different pairs of variables, leading to significant outcomes based on their respective P values being less than the level of significance (<0.05) for each pair of variables being tested.
For testing the association between continuous variables that are not symmetric but skewed in nature, the non-parametric Kruskal–Wallis test was used. The statistical significance is established at a 5% level of significance. We performed a univariate and multivariate binomial logistic regression analysis to study and estimate the influence of various risk factors associated with AKI. The corresponding model led to the estimation of the adjusted odds ratio and their respective 95% confidence intervals.
We used univariable and multivariable cox regression models with AKI as the time-varying exposure and in-hospital mortality as the outcome to assess the association between AKI and in-hospital death. Pre-selected models were created using known risk variables for AKI-related mortality in hospitalized patients.[1718] Patients were censored on the day of discharge. Risk differences and age-adjusted odds ratios, as well as 99.5% confidence intervals, were used to compare COVID-19 outcomes between the first and second waves. The quantitative data of 1260 patients were collected, with data being collected with respect to different nominal variables; some of the laboratory data were lost/missing (<3%). Multiple imputations are used as a general approach to the problem of missing data available in the given dataset. Analysis was performed on IBM SPSS Statistics for Windows Version 28.0.0.
Results
Baseline characteristics
A total of 1260 patients with COVID-19 were included in our cohort. The median age was 56 (IQR: 47–66) years, and 63.6% were men (n = 801). The median duration from illness onset to admission was 6 days. Diabetes mellitus was the most frequent comorbidity (55.2%), followed by hypertension (42.1%), hypothyroidism (11.3%), and coronary artery disease (8.1%). Fever was the most common presenting symptom (84.4%), followed by cough (66.3%), fatigue (50.3%), and diarrhea (17.1%). A total of 229 (18.17%) patients were admitted in ICU, 574 (45.5%) received ventilation, 26 (2.0%) required invasive mechanical ventilation, and 36 (2.9%) patients died. Patients requiring renal replacement therapy (RRT) were 0.6% (n = 8). The median length of hospital stay was 6 days (IQR: 5–8). CT scan revealed >30% involvement in 80% (n = 48) patients with AKI.
Determinants and outcomes of kidney disease
According to their AKI status, the demographic variables of individuals are expressed in Table 1. Among 1260 patients, 6.8% (n = 86) patients developed AKI: 57 patients with stage 1, 20 with stage 2, and 9 with stage 3 AKI. Ninety-five percent of the patients who developed AKI were aged >50 years. Because the median period from sickness to admission was 6 days, 98% of the patients presented with AKI on the day of admission. Urine analysis was available in 65.5% of the patients. Leukocyturia was seen in 53.4% of patients with AKI compared to 20.6% without AKI; microscopic hematuria was observed in 15.5% of patients with AKI compared to 6.1% without AKI. The median admission creatinine for patients without AKI was 0.83 mg/dL (IQR: 0.70–1.00), with AKI stage 1 was 1.66 mg/dL (IQR: 1.55–1.90), AKI stage 2 was 2.73 mg/dL (IQR: 2.50–3.11), and AKI stage 3 was 5.1 mg/dL (IQR: 4.60–5.63).
| Variables | Overall | No AKI | AKI-1 | AKI-2 | AKI-3 | P |
|---|---|---|---|---|---|---|
| n=1260 | n=1174 | n=57 | n=20 | n=9 | 0.001 | |
| Age | ||||||
| <40 | 175 (13.9%) | 173 (14.7%) | 0 (0%) | 2 (10%) | 0 (0%) | |
| 40-49 y | 223 (17.7%) | 218 (18.6%) | 4 (7%) | 1 (5%) | 0 (0%) | |
| 50-59 y | 335 (26.6%) | 323 (27.5%) | 9 (15.8%) | 2 (10%) | 1 (11.1%) | |
| 60-69 y | 304 (24.1%) | 276 (23.5%) | 17 (29.8%) | 7 (35%) | 4 (44.4%) | |
| 70-79 y | 164 (13%) | 135 (11.5%) | 18 (31.6%) | 7 (35%) | 4 (44.4%) | |
| >=80 y | 58 (4.6%) | 49 (4.1%) | 9 (15.8%) | 1 (5%) | 0 (0%) | |
| Gender no: (%) | 0.10 | |||||
| Female | 459 (36.4%) | 437 (37.2%) | 14 (24.6%) | 7 (35%) | 1 (11.1%) | |
| Male | 801 (63.6%) | 737 (62.8%) | 43 (75.4%) | 13 (65%) | 8 (88.9%) | |
| Co-morbidities no (%) | ||||||
| Hypertension | 531 (42.1%) | 472 (40.2%) | 40 (70.2%) | 16 (80%) | 3 (33.3%) | 0.00 |
| Diabetes | 695 (55.2%) | 635 (54.1%) | 41 (71.9%) | 14 (70%) | 5 (55.6%) | 0.03 |
| Chronic Lung disease | 65 (5.2%) | 58 (4.9%) | 4 (7%) | 2 (10%) | 1 (11.1%) | 0.24 |
| Interstitial Lung disease | 8 (0.6%) | 7 (0.6%) | 1 (1.8%) | 0 (0%) | 0 (0%) | 0.43 |
| Congestive Heart failure | 14 (1.1%) | 11 (0.9%) | 2 (3.5%) | 1 (5%) | 0 (0%) | 0.09 |
| Ischemia Heart disease | 25 (1.9%) | 19 (1.6%) | 6 (10.5%) | 0 (0%) | 0 (0%) | 0.00 |
| Cancer | 13 (1%) | 11 (0.9%) | 2 (3.5%) | 0 (0%) | 0 (0%) | 0.26 |
| Hypothyroidism | 142 (11.3%) | 132 (11.3%) | 7 (12.3%) | 3 (15%) | 0 (0%) | 0.74 |
| Autoimmune disease | 24 (1.9%) | 20 (1.7%) | 2 (3.5%) | 2 (10%) | 0 (0%) | 0.06 |
| Dyslipidaemia | 53 (4.2%) | 44 (3.8%) | 6 (10.5%) | 3 (15%) | 0 (0%) | 0.01 |
| Tuberculosis | 9 (0.7%) | 8 (0.7%) | 1 (1.8%) | 0 (0%) | 0 (0%) | 0.47 |
| Stroke | 19 (1.5%) | 16 (1.4%) | 1 (1.8%) | 1 (5%) | 1 (11.1%) | 0.05 |
| Coronary artery disease | 102 (8.1%) | 82 (7%) | 14 (24.6%) | 4 (20%) | 2 (22.2%) | 0.00 |
| Chronic liver disease | 12 (0.9%) | 10 (0.9%) | 1 (1.8%) | 1 (5%) | 0 (0%) | 0.17 |
| Hyperthyroidism | 7 (0.5%) | 7 (0.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Seizure disorder | 6 (0.4%) | 5 (0.4%) | 0 (0%) | 0 (0%) | 1 (11.1%) | 0.09 |
| Iron deficiency anaemia | 7 (0.4%) | 6 (0.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Severity of illness | ||||||
| ICU n (%) | 229 (18.17%) | 182 (14.1%) | 24 (42.1%) | 15 (75%) | 8 (88.88%) | 0.01 |
| Invasive mechanical ventilation n (%) | 26 (2.0%) | 20 (1.7%) | 3 (5.3%) | 2 (10%) | 1 (11.1%) | 0.01 |
| Renal replacement therapy, n % | 8 (88.88%) | 0.001 | ||||
| Extracorporeal membrane oxygenation (ECMO), n(%) | 3 (0.2%) | 1 (0.1%) | 1 (1.8%) | 1 (5%) | 0 (0%) | 0.01 |
| Variables | Overall | No AKI | AKI-1 | AKI-2 | AKI-3 | P |
| Severity of illness | ||||||
| Total number of patients requiring ventilation n (%) | 574 (45.5%) | 512 (43.6%) | 35 (61.4%) | 18 (90%) | 9 (100%) | 0.001 |
| Length of ICU stay, n, (IQR), median | 229 (4-11) 7 | 182 (4-10) 6 | 24 (3-11) 6 | 15 (5-12) 8 | 8 (6-12) 9 | 0.42 |
| Admission SpO2% n, (IQR), median | 1258 (92-97) 96 | 1172 (92-97) 96 | 57 (88-96) 94 | 20 (74-96) 86 | 9 (82-92) 85 | 0.00 |
| Severity of COVID-19, n (%) | ||||||
| Mild | 876 (69.5%) | 839 (71.4%) | 28 (49.1%) | 8 (40%) | 0 (0%) | 0.0001 |
| Moderate | 215 (17.06%) | 196 (16.69%) | 15 (26.31%) | 3 (15%) | 1 (11.11%) | 0.0001 |
| Severe | 169 (13.41%) | 139 (11.91%) | 14 (24.56%) | 9 (45%) | 8 (88.88%) | 0.0001 |
Within the study population, 36 (2.9%) died and 1224 (97.1%) were discharged at the time of the study. The incidence of in-hospital death in the patients with AKI was nine in stage 1 (15.8%), seven in stage 2 (35%), and five in stage 3 (55.6%), which was significantly higher than those with normal admission serum creatinine 15 (1.3%) [Figure 2]. Among eight patients who required RRT, four (50%) died in-hospital. In-hospital mortality of patients with AKI was 24.4% versus 1.3% in patients without AKI. Among a total of 229 (18.17%) admitted to ICU, individuals with AKI were 42%, 75%, and 88% as per the stages, 1, 2 and 3 respectively. When compared to those who did not develop AKI, patients with AKI were more likely to be admitted to intensive care units (54.65% vs. 15.5%) and receive mechanical ventilation (72.09% vs. 43.6%). Patients who developed AKI were older and had a higher prevalence of comorbidities, including hypertension, diabetes mellitus, coronary artery disease, dyslipidemia, and heart failure than patients without AKI. Table 2 and Figure 3 show that patients with AKI had a more severe illness as shown by markedly raised serum inflammatory markers such as D-dimer, C-reactive protein, lactate dehydrogenase, interleukin-6, and ferritin.
| Variables | Overall | No AKI | AKI-1 | AKI-2 | AKI-3 | P |
|---|---|---|---|---|---|---|
| Laboratory test results within 48 h of hospital admission | n=1260 | n=1174 | n=57 | n=20 | n=9 | |
| WBC count (x 109/L) n, (IQR), median | 1257 (5.19-9.88) 6.83 | 1171 (5.10-9.56) 6.73 | 57 (5.97-12.77) 8.69 | 20 (8.6-19.83) 15.73 | 9 (11.3-31.47) 19.56 | 0.001 |
| Admission serum creatinine (mg/dl) n, (IQR), median | 1260 (0.71-1.04) 0.85 | 1174 (0.70-1.00) 0.83 | 57 (1.55-1.90) 1.66 | 20 (2.50-3.31) 2.73 | 9 (4.60-5.63) 5.1 | 0.001 |
| Discharge serum creatinine (mg/dl) n, (IQR), median | 447 (0.65-0.94) 0.78 | 395 (0.64-0.88) 0.75 | 33 (0.86-1.27) 1.06 | 15 (1.19-1.62) 1.39 | 4 (1.41-1.9) 1.41 | 0.00001 |
| Albumin (g/dl) n, (IQR), median | 1257 (3.6-4.2) 3.9 | 1171 (3.6-4.2) 3.9 | 57 (3.2-4.0) 3.7 | 20 (2.8-3.6) 3.1 | 9 (2.4-3.2) 2.9 | 0.001 |
| D-Dimer ng/ml n, (IQR), median | 1257 (136.0-408.5) 213.0 | 1171 (134-370.2) 203.5 | 57 (183.5-1044.5) 353.0 | 20 (342.5-3091.2) 542.0 | 9 (374.5-2988.5) 603.0 | 0.001 |
| C-Reactive protein (mg/L) n, (IQR), median | 1257 (11.37-86.19) 36.96 | 1171 (10.3-81.6) 34.2 | 57 (29.2-138.2) 78.0 | 20 (46.7-259.5) 134.2 | 9 (115.8-202.6) 149.2 | 0.001 |
| Ferritin, (microg/L) n, (IQR), median | 1257 (122.15-523.45) 265.45 | 1171 (118.3-509.0) 259.8 | 57 (156.75-769.95) 315.0 | 20 (337.4-961.4) 562.9 | 9 (402.1-1408.5) 1156.0 | 0.001 |
| Interleukin-6 pg/ml n, (IQR), median | 1029 (9.11-58.42) 24.90 | 960 (8.7-54.8) 23.1 | 44 (19.5-144.3) 47.6 | 16 (30.3-322.0) 117.9 | 9 (121.5-3099.0) 1156.0 | 0.001 |
| Lactate Dehydrogenase U/L n, (IQR), median | 1038 (202.77-329.50), 252.45 | 963 (200.00-321.00) 249.70 | 49 (222.90-350.45) 271.70 | 18 (319.67-597.22) 361.35 | 8 (244.47-699.45) 320.75 | 0.001 |
| Blood urea (mg/dl) n, (IQR), median | 1257 (18.80-37.95) 25.70 | 1171 (18.5-34.9) 24.8 | 57 (41.20-79.8) 60.10 | 20 (59.6-108.05) 84.7 | 9 (126.5-145.40) 176.35 | 0.001 |
| Blood Sugar (mg/dl) n, (IQR), median | 1257 (117.65-224.65) 155.30 | 1171 (117.00-222.90) 153.90 | 57 (113.20-228.90) 158.80 | 20 (152.65-240.40) 200.40 | 9 (142.50-382.65) 223.80 | 0.027 |
| Total Bilirubin (mg/dl) n, (IQR), median | 1257 (0.31-0.63) 0.44 | 1171 (0.31-0.63) 0.43 | 57 (0.39-0.78) 0.53 | 20 (0.40-0.85) 0.58 | 9 (0.57-4.06) 1.18 | 0.001 |
| Alanine aminotransferase U/L n, (IQR), median | 1257 (16.70-39.95) 25.10 | 1171 (16.60-39.30) 24.80 | 57 (16.80-49.30) 24.70 | 20 (26.60-46.30) 101.32 | 9 (30.60-443.75) 46.10 | 0.001 |
| Alkaline phosphatase U/L n, (IQR), median | 1257 (56.40-89.00) 70.00 | 1171 (56.14-87.86) 69.68 | 57 (57.60-94.75) 70.70 | 20 (74.37-131.70) 100.65 | 9 (63.45-300.90) 104.30 | 0.001 |
| Aspartate aminotransferase U/L n, (IQR), median | 1257 (21.15-45.0) 29.80 | 1171 (21.00-43.80) 29.20 | 57 (24.05-54.50) 37.00 | 20 (28.65-142.57) 63.30 | 9 (50.40-432.00) 80.60 | 0.001 |
| Urine WBC n, (%) | 0.001 | |||||
| Nil | 15 (1.2%) | 12 (1%) | 2 (3.5%) | 1 (5%) | 0 (0%) | |
| <2-4 hpf | 538 (42.7%) | 514 (43.8%) | 15 (26.3%) | 9 (45%) | 0 (0%) | |
| >4-6 hpf | 273 (21.7%) | 242 (20.6%) | 22 (38.6%) | 3 (15%) | 6 (66.7%) | |
| Not done | 434 (35.5%) | 406 (35.4%) | 18 (31.5%) | 8 (35%) | 3 (33.3%) | |
| Urine RBC n, (%) | 0.001 | |||||
| Nil | 525 (41.7%) | 497 (42.4%) | 23 (40.4%) | 3 (15%) | 2 (22.2%) | |
| <2-4 hpf | 214 (17%) | 199 (17%) | 9 (15.8%) | 4 (20%) | 2 (22.2%) | |
| >4-6 hpf | 87 (6.9%) | 72 (6.1%) | 7 (12.3%) | 6 (30%) | 2 (22.2%) | |
| Not done | 434 (35.5%) | 406 (35.4%) | 18 (31.5%) | 8 (35%) | 3 (33.3%) | |
| Urine protein n (%) | 0.001 | |||||
| 1+ | 267 (21.2%) | 246 (21%) | 18 (31.6%) | 2 (10%) | 1 (11.1%) | |
| 2+ | 129 (10.2%) | 110 (9.4%) | 10 (17.5%) | 5 (25%) | 4 (44.4%) | |
| 3+ | 45 (3.6%) | 37 (3.2%) | 4 (7%) | 3 (15%) | 1 (11.1%) | |
| 4+ | 12 (1%) | 11 (0.9%) | 0 (0%) | 1 (5%) | 0 (0%) | |
| Nil | 373 (29.7%) | 364 (31.1%) | 7 (12.3%) | 2 (10%) | 0 (0%) | |
| eGFR from on admission Scr n (%) | 0.001 | |||||
| >=60 ml/min/1.73 m2 | 1059 (84%) | 1059 (90.2%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 45-59 ml/min/1.73 m2 | 109 (8.65%) | 104 (8.9%) | 5 (8.8%) | 0 (0%) | 0 (0%) | |
| 30-45 ml/min/1.73 m2 | 56 (4.44%) | 11 (0.9%) | 45 (78.9%) | 0 (0%) | 0 (0%) | |
| 15-29 ml/min/1.73 m2 | 24 (1.9%) | 0 (0%) | 7 (12.3%) | 17 (85%) | 0 (0%) | |
| <15ml/min/1.73 m2 | 12 (0.95%) | 0 (0%) | 0 (0%) | 3 (15%) | 9 (100%) | |
| Imaging n (%) | 0.001 | |||||
| Patients with no available CT reports | 290 (23%) | 264 (22.4%) | 17 (29.8%) | 5 (25%) | 4 (44.44%) | |
| Patients with CT reports and CT score<8 (mild infection) | 302 (23.9%) | 290 (24.7%) | 6 (10.5%) | 6 (30%) | 0 (0%) | |
| CT score 8-17 (moderate infection) | 498 (39.5%) | 475 (40.5%) | 19 (33.33%) | 2 (10%) | 2 (22.22%) | |
| CT score ≥18 (severe infection) | 170 (13.4%) | 145 (12.3%) | 15 (26.3%) | 7 (35%) | 3 (33.33%) | |
| Symptoms | ||||||
| Fever n, (IQR), median | 1064 (3-7) 5 | 1006 (3-7) 5 | 44 (3-7) 4 | 11 (2-8) 5 | 3 (1-7) 4 | 0.549 |
| SOB n, (IQR), median | 452 (1-3) 2 | 410 (1-3) 2 | 22 (1-5) 2 | 14 (1-3) 2 | 6 (1-2.50) 2 | 0.966 |
| Fatigue n, (IQR), median | 635 (3-6) 4 | 605 (3-6) 4 | 23 (3-5) 3 | 6 (3.50-7.75) 6.50 | 8 (1-1) 1 | 0.156 |
| Loss of taste n, (IQR), median | 195 (15.5%) | 182 (15.5%) | 10 (17.5%) | 3 (15.5%) | 0 (0%) | 0.001 |
| Loss of smell n, (IQR), median | 274 (21.7%) | 260 (22.1%) | 12 (21.1%) | 2 (10%) | 0 (0%) | 0.286 |
| Cough n, (IQR), median | 836 (3-7) 4 | 791 (3-7) 4 | 36 (2-5) 3 | 5 (4-10) 7 | 4 (2.50-4.75) 4 | 0.076 |
| Diarrhoea, n % | 216 (17.2%) | 203 (17.3%) | 8 (14%) | 4 (20%) | 1 (11.1%) | 0.891 |
| Loss of appetite, n % | 84 (6.7%) | 79 (6.7%) | 3 (5.3%) | 1 (5.0%) | 1 (11.1%) | 0.749 |
| Vomiting, n % | 68 (5.4%) | 60 (5%) | 4 (7%) | 2 (10%) | 2 (22.2%) | 0.07 |
| Abdominal pain, n % | 16 (1.3%) | 13 (1.1%) | 2 (3.5%) | 0 (0%) | 1 (11.1%) | 0.05 |
| Complications n% | N | |||||
| Respiratory failure | 124 (9.8%) | 111 (9.5%) | 7 (12.3%) | 4 (20%) | 2 (22.2%) | 0.127 |
| Sepsis | 28 (2.2%) | 17 (1.4%) | 4 (7%) | 3 (15%) | 4 (44.4%) | 0.001 |
| Multiorgan dysfunction | 10 (0.8%) | 3 (0.3%) | 2 (3.5%) | 3 (15%) | 2 (22.2%) | 0.001 |
| Pulmonary thromboembolism | 9 (0.7%) | 8 (0.7%) | 1 (1.8%) | 0 (0%) | 0 (0%) | 0.472 |
| Pneumothorax | 6 (0.5%) | 5 (0.4%) | 0 (0%) | 1 (5%) | 0 (0%) | 0.155 |
| Cardiogenic shock | 1 (0.1%) | 1 (0.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.493 |
| Heparin induced thrombocytopenia | 3 (0.2%) | 2 (0.2%) | 1 (1.8%) | 0 (0%) | 0 (0%) | 0.191 |
| Severe acute respiratory syndrome | 20 (1.6%) | 17 (1.4%) | 2 (3.5%) | 0 (0%) | 1 (11.1%) | 0.103 |
| Septic shock | 6 (0.5%) | 3 (0.3%) | 2 (3.5%) | 1 (5%) | 0 (0%) | 0.006 |
| Death (Fatal event) | 36 (2.9%) | 15 (1.3%) | 9 (15.8%) | 7 (35%) | 5 (55.6%) | 0.001 |
| Length of hospital stay. n, (IQR), median |
1256 (5-8) 6 | 1170 (5-8) 6 | 57 (5-12.50) 8 | 20 (6-14.25) 9 | 9 (5.50-14.50) 11 | 0.001 |
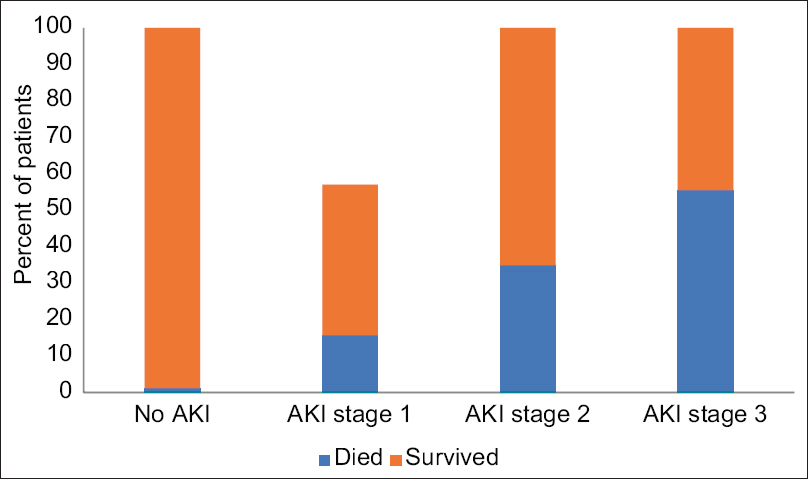
- Bar diagram showing mortality as per the stages of AKI. The all-cause mortality was 2.9%. (AKI stage 1: 15.78%; stage 2: 35%; stage 3: 55.55%), (No AKI: 1.2%)
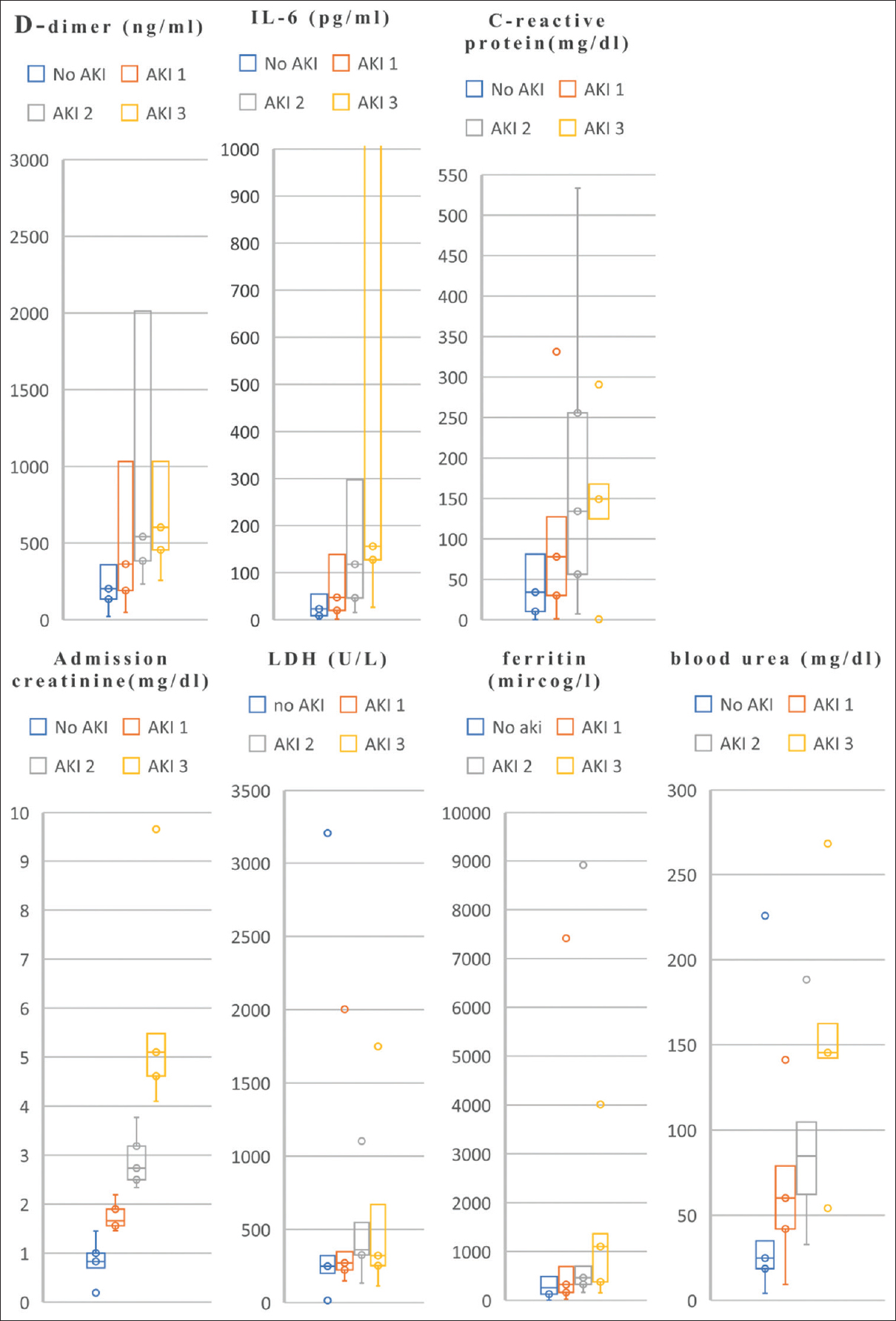
- Inflammatory markers as per stages of AKI and no AKI. Note: Box plots (median, IQR, and whiskers denoting 5th and 95th percentiles). Most plots do not have upper whiskers due to outliers. In patients with COVID-19, less than 3% of variables are missing, except IL-6 and LDH, which were selectively done in 82% of the population
Univariate analysis showed that male gender, diabetes mellitus, hypertension, coronary artery disease, dyslipidemia, C-reactive protein, and d-dimer were associated with AKI development [Table 3]. Independent risk factors for AKI were hypertension (odds ratio (OR): 3.250, CI: 2.031–5.201), diabetes mellitus (OR: 1.959, CI: 1.219–3.148), coronary artery disease (OR: 4.035, CI: 2.332–6.983), dyslipidemia (OR: 3.002, CI: 1.413–6.376), C-reactive protein (OR: 3.668, CI: 1.674–8.037), and d-dimer (OR: 4.206, CI: 2.573–6.877).
| Variables | Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| P | Odds ratio | Lower confidence interval | Upper confidence interval | P | Odds ratio | Lower confidence interval | Upper confidence interval | |
| Gender (Female vs. male) | 0.032 | 0.580 | 0.352 | 0.955 | ||||
| DM (Yes vs. No) | 0.005 | 1.959 | 1.219 | 3.148 | ||||
| Hypertension (Yes vs. No) | <0.001 | 3.250 | 2.031 | 5.201 | 0.008 | 1.98 | 1.192 | 3.292 |
| Hypothyroidism (Yes vs. No) | 0.913 | 1.039 | 0.524 | 2.058 | ||||
| Coronary artery disease (Yes vs. No) | <0.001 | 4.035 | 2.332 | 6.983 | 0.001 | 2.656 | 1.469 | 4.802 |
| Chronic lung disease (Yes vs. No) | 0.200 | 1.705 | 0.753 | 3.858 | ||||
| Heart failure (Yes vs. No) | 0.056 | 3.500 | 0.969 | 12.646 | ||||
| Ischemic heart disease (Yes vs. No) | 0.002 | 4.559 | 1.771 | 11.735 | 0.0011 | 4.365 | 1.409 | 13.521 |
| Cancer (Yes vs. No) | 0.235 | 2.517 | 0.549 | 11.542 | ||||
| Autoimmune (Yes vs. No) | 0.064 | 2.815 | 0.940 | 8.427 | ||||
| Dyslipidaemia (Yes vs. No) | 0.004 | 3.002 | 1.413 | 6.376 | 0.063 | 2.243 | 0.958 | 5.248 |
| Tuberculosis (Yes vs. No) | 0.613 | 1.715 | 0.212 | 13.870 | ||||
| Requiring Ventilation (Yes vs. No) | <0.001 | 3.063 | 1.884 | 4.978 | 0.097 | 1.595 | 0.920 | 2.766 |
| C-reactive protein, mg/dl (≥10 vs. <10) | <0.001 | 3.668 | 1.674 | 8.037 | 0.141 | 1.874 | 0.812 | 4.326 |
| D-dimer, pg/L (≥250 vs. <250) | <0.001 | 4.206 | 2.573 | 6.877 | <0.001 | 2.796 | 1.642 | 4.762 |
| Blood Urea, mg/dl (≥20 vs. <20) | <0.001 | 12.244 | 2.2992 | 50.100 | 0.011 | 6.368 | 1.522 | 26.645 |
In multivariate analysis, hypertension, coronary artery disease, and elevated d-dimer were the only risk factors for AKI. [Figure 4] shows the findings of a Kaplan–Meier survival analysis stratified by AKI status. Using the group without AKI as the reference in Supplementary Table 1, the unadjusted hazard ratio for in-hospital death was 9.469 (95% CI: 4.735–18.935, P < 0.001) in the AKI 1–3 group, and after accounting for possible confounders in different models, the association remained statistically significant.
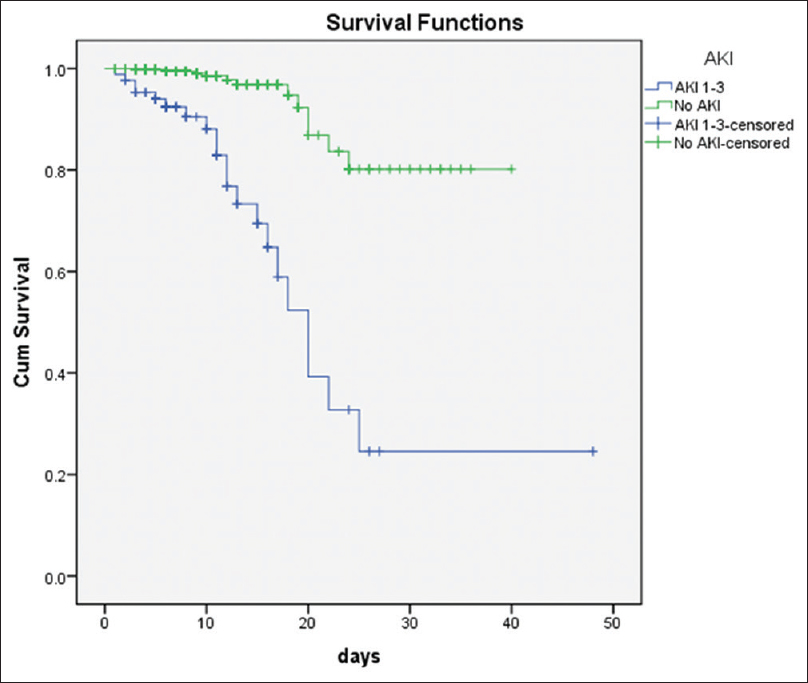
- Survival analysis shows higher in hospital mortality in patients with COVID-19 infection
| AKI 1-3 | |||
|---|---|---|---|
| Hazard Ratio (HR) | 95% Confidence Interval | P | |
| Unadjusted | 9.469 | (4.735-18.935) | <0.001 |
| Adjusted with model 1 | 8.249 | (3.956-17.204) | <0.001 |
| Adjusted with model 2 | 7.433 | (3.529-15.654) | <0.001 |
| Adjusted with model 3 | 17.499 | (5.505-55.621) | <0.001 |
Abbreviations: AKI, acute kidney injury; AKI 1-3, AKI stages 1-3; HR, hazard ratio. Note: Time-varying Cox proportional hazard models showing association of COVID-19 with Acute kidney Injury. The reference used was no AKI status. Model 1: Includes adjusted demographic, including age, sex. Model 2: Includes model 1+ adjusted for comorbidities (diabetes, Hypertension, Coronary artery disease, Hypothyroidism, Ischemic Heart disease, Dyslipidaemia, Chronic lung disease, Heart failure, Cancer, Autoimmune diseases. Model 3: Includes model 2+ values of oxygen saturation, serum WBC >10.0, serum urea >18, serum bilirubin, serum albumin, C-reactive protein >10, serum ferritin, D-dimer >250, and the need for mechanical ventilation
Comparison of COVID-19 outcomes
The first and second wave outcomes are compared in Table 4. Compared to the first wave, the second wave cohort had a lower risk for AKI (adj OR: 0.426; CI: 0.232–0.782), mortality (adj OR: 0.252; CI: 0.090–0.707), sepsis (adj OR: 0.130; CI: 0.029–0.570), requirement of invasive mechanical ventilation (adj OR: 0.234; CI: 0.067–0.819), and length of ICU stay >5 days (adj OR: 0.446; CI: 0.267–0.745). Patient demographic characteristics and comorbidities for the first and second wave COVID-19 cohorts are presented in Supplementary Table 2. The second wave cohort was younger (mean age standard deviation = 54.4 ± 14.48 years) than the first wave (mean age standard deviation = 56.86 ± 14.05 years) and had proportionally fewer preexisting diseases, such as diabetes mellitus, hypertension, and coronary artery disease. Compared to the first wave, a smaller proportion of second wave patients had laboratory values beyond the normal range Supplementary Table 3].
| Clinical outcomes | First wave (n=748) | Second wave (n=512) | Second wave vs. First wave | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absolute risk n (%) | Absolute risk n (%) | Risk difference | 99.5% CI | Age-adjusted odds ratio | 99.5% CI | |||||
| Total number of patients requiring invasive mechanical ventilation | 20 | 2.67% | 6 | 1.17% | −0.0150 | −0.0546 | 0.0245 | 0.2346 | 0.0672 | 0.8191 |
| Total number of patients requiring ventilation | 352 | 47.06% | 222 | 43.36% | −0.0370 | −0.0765 | 0.0025 | 0.7848 | 0.5701 | 1.0802 |
| Length of ICU stays >5 days | 96 | 12.83% | 44 | 8.59% | −0.0424 | −0.0819 | −0.0029 | 0.4466 | 0.2677 | 0.7450 |
| Respiratory failure | 65 | 8.69% | 59 | 11.52% | 0.0283 | −0.0112 | 0.0679 | 0.8477 | 0.5217 | 1.3775 |
| Sepsis | 24 | 3.21% | 4 | 0.78% | −0.0243 | −0.0638 | 0.0153 | 0.1303 | 0.0297 | 0.5709 |
| Acute Kidney Injury (AKI) | 56 | 7.49% | 30 | 5.86% | −0.0163 | −0.0558 | 0.0233 | 0.4267 | 0.2328 | 0.7823 |
| Length of Hospital Stay >3 days | 628 | 83.96% | 465 | 90.82% | 0.0686 | 0.0291 | 0.1082 | 2.2569 | 1.3669 | 3.7263 |
| Death (fatal event) | 27 | 3.61% | 9 | 1.76% | −0.0185 | −0.0581 | 0.0210 | 0.2528 | 0.0903 | 0.7079 |
| Demographics | First wave | Second wave | P | ||
|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | ||
| Age in years, mean±SD | 56.86 | 14.05 | 54.44 | 14.48 | 0.0031 |
| Gender | N | % | N | % | |
| Male, n (%) | 487 | 38.70% | 314 | 24.90% | 0.1960 |
| Female, n (%) | 262 | 20.80% | 197 | 15.60% | |
| Comorbidities | N | % | N | % | |
| Hypertension | 325 | 25.80% | 206 | 16.30% | 0.2770 |
| Diabetes | 391 | 31.00% | 304 | 24.10% | 0.0110 |
| Chronic lung disease | 36 | 2.90% | 29 | 2.30% | 0.4940 |
| Hypothyroidism | 84 | 6.70% | 58 | 4.60% | 0.9410 |
| Coronary artery disease | 64 | 5.10% | 38 | 3.00% | 0.4790 |
| Heart failure | 12 | 1.00% | 3 | 0.20% | 0.1030 |
| Ischemic Heart disease | 10 | 0.80% | 15 | 1.20% | 0.0450 |
| Dyslipidemia | 24 | 1.90% | 29 | 2.30% | 0.0320 |
| Laboratory values | Exceeded upper limit of normal n (%) | Second wave versus the first wave | ||||||
|---|---|---|---|---|---|---|---|---|
| First wave | Second wave | Difference | 99.5% CI | |||||
| n | % | n | % | n | % | |||
| WBC count >10/L | 163 | 12.94% | 111 | 8.81% | −52 | -4.13% | −8.08% | −0.17% |
| Serum creatinine ≥1.17 mg/dl | 133 | 10.56% | 71 | 5.63% | −62 | −4.92% | −8.87% | −0.97% |
| D-Dimer ≥250 ng/ml | 306 | 24.29% | 205 | 16.27% | −101 | −8.02% | −11.97% | −4.06% |
| C-reactive ≥10 mg/dl | 547 | 43.41% | 404 | 32.06% | −143 | −11.35% | −15.30% | −7.40% |
| Interleukin - 6 ≥10 pg/ml | 449 | 35.63% | 303 | 24.05% | −146 | −11.59% | −15.54% | −7.63% |
| Lactate dehydrogenase ≥280 U/L | 232 | 18.41% | 166 | 13.17% | −66 | −5.24% | −9.19% | −1.28% |
| Blood urea ≥20 mg/dl | 505 | 40.08% | 350 | 27.78% | −155 | −12.30% | −16.26% | −8.35% |
| Bilirubin ≥1.2 mg/dl | 26 | 2.06% | 16 | 1.27% | −10 | −0.79% | −4.75% | 3.16% |
| Alanine aminotransferase ≥40 U/L | 167 | 13.25% | 102 | 8.10% | −65 | −5.16% | −9.11% | −1.20% |
| Aspartate aminotransferase ≥38 U/L | 237 | 18.81% | 155 | 12.30% | −82 | −6.51% | −10.46% | −2.55% |
| Alkaline phosphatase ≥100 U/L | 125 | 9.92% | 49 | 3.89% | −76 | −6.03% | −9.99% | −2.08% |
Discussion
India, the second-most populous country with the second-largest burden of COVID-19 and a case fatality rate of 1.3%, has witnessed immense pressure on the healthcare system with partial collapse of the infrastructure. AKI has been reported as a severe complication of COVID-19 with a higher risk of mortality.[19] There are isolated reports of AKI due to the unmonitored use of herbal therapies to prevent COVID-19. However, there may be many more such episodes that were not diagnosed and reported in COVID-19 infected patients.[20] As there is a scarcity of information on AKI in COVID-19 patients in India, this retrospective study was undertaken in a tertiary healthcare private facility, looking at the diagnosis and management of AKI during the first wave and part second wave COVID-19. A 6.8% prevalence of AKI was reported in our study of patients hospitalized with COVID-19.
The reported incidence of AKI as between 1% and 42% among those infected with COVID-19 is currently only based on reports from small case series and retrospective studies from China,[21] Europe,[2223] the United States,[19] and India.[121324] The variances between previous studies and our investigation might be related due to variances in patient populations and geographic location. Our AKI rates are consistent with other recent studies from India,[1213] which found that AKI incidence is between 7 and 7.2%. Due to the high case burden in India, as hospitals were choked with patients, they were advised to stay at home until the oxygen saturation measured by oximeter fell below 94%; this probably led to the increased case burden of AKI at admission. Volume depletion at admission might be expected in patients with COVID-19 as they typically present with fever and pre-hospital fluid resuscitation is rarely performed. We found that 98% of the patients presented with AKI on the day of admission. Hypovolemia treatment is cost-effective and should be implemented to prevent AKI. The management varied regarding ventilation, hemodynamic support, RRT, ECMO, and drug therapy as there was limited knowledge of steroids, remdesivir, anti-microbial, and anti-fungal drugs.
In our study, among patients who developed AKI, 54.6% required ICU care, 72.09% required ventilation, and 24.4% died. Thus, our patients overall mortality rate in AKI is consistent with the study conducted by Sindhu and Prasad[12] but is lower than other studies[1325] that reported mortality rates between 35% and 45%. This might be due to the fact that more patients in our study had a lower stage of AKI than those in other studies.
Several mechanisms are possible for AKI in COVID-19 patients, including multi-organ dysfunction syndrome, AKI following acute respiratory distress syndrome (ARDS), ischemic acute tubular injury, and cytokine storm syndrome. In addition, a recently published study that utilized autopsy specimens from 26 patients who died of COVID-19 in China demonstrated that there is evidence of the invasion of COVID-19 into kidney tissue, along with significant acute tubular injury and endothelial damage, as well as glomerular and vascular changes indicative of underlying diabetic or hypertensive disease.[26] Unfortunately, we have not done kidney biopsies or post-mortem studies in our cohort with AKI, although various biomarkers and urinary findings either alone or in combination suggested AKI.
The accurate diagnosis of AKI is especially problematic in critically ill patients with unsteady kidney function; the validity of creatinine-based baseline assessment measures has limitations. As a result, using a biomarker kit that includes multiple early indicators with various features is more accurate. However, cost constraints limit their use as in our analysis. In the absence of specific treatment options, the care strategy for patients with COVID-19 in the ICU remains largely supportive. Implementation of the kidney disease: Improving Global Outcomes (KDIGO) supporting care guidelines such as avoiding nephrotoxins, regular monitoring of serum creatinine and urine output, and hemodynamic monitoring in critically ill patients with kidney involvement is likely to reduce the occurrence and severity of AKI in COVID-19. Conservative management of volume overload, metabolic acidosis, and hyperkalemia can be attempted before considering initiation of dialysis. The validity of early dialysis, either hemodialysis or peritoneal dialysis, for removing inflammatory markers is still questionable. RRT should be considered if conservative management fails in patients with volume overload, especially those with refractory hypoxemia. Many centers in developed and developing countries reported using acute peritoneal dialysis as a safe and cost-effective RRT in COVID-19-related AKI.[27] In our study, the mean discharge creatinine of patients who required RRT was 1.75 mg/dL. As long-term renal prognosis is unknown, these patients should be followed up periodically for CKD diagnosis.
Similarly, there is insufficient data in India comparing the outcomes of the first and second waves of the COVID-19. Our study shows that the second wave of COVID-19 in Chennai, India exhibited improved clinical outcomes compared to the first wave. A study using a Japanese public registry[28] of 5194 patients discovered that the second wave had fewer comorbidities, younger demographics, and lower mortality. A study from Spain[29] of 468 hospitalized COVID-19 patients found similar outcomes with less need for invasive mechanical ventilation, conventional oxygen therapy, and lower mortality in the second wave. A recent large study done by Hoogenboom[30] exhibited superior results across all age, racial, and ethnic groups as compared to the first wave. Our findings are consistent with the other studies that the second wave had better outcomes. In addition, the differences in outcomes between the first and second waves could also be due to learnings from the first wave, which led to better management of COVID-19 patients by providing prioritised treatment. However, more extensive multicentre studies are needed to be conducted in India to look for accurate outcome measures.
Limitations of our study
This retrospective analysis has several limitations. 1) As we do not have previous serum creatinine levels, we used the upper limit of normal creatinine level (104 μ/L or 1.17 mg/dL) values for the diagnosis, which might have resulted in an underestimation of AKI. 2) The incidence of AKI detection may also be affected by the frequency of serum creatinine estimation. 3) Details of previous home medications, including angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, vancomycin, diuretics, and nonsteroidal anti-inflammatory drugs, were not available in all patients. 4) Because the research group was limited to a particular location, the findings may not apply to all patients with AKI across India.
In conclusion, AKI prevalence in our study population was 6.8%, and mortality occurred in 24.4% of patients with AKI. Risk factors for AKI include hypertension, diabetes, coronary artery disease, dyslipidemia, need for ventilation, and higher markers of inflammation. Our study demonstrates that the second wave had better clinical outcomes compared to the first wave of COVID-19 in Chennai, India.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The doctors are grateful for the efforts of the entire hospital team in treating patients during the pandemic. The study was designed by Prof. Georgi Abraham and Dr. ShreevidyaVenkatraman. Data collection and preparation of tables and figures were done by Dr. Phanidhar Mogga and Dr. Georgi Abraham. Statistical analysis was done by Dr. Phanidhar Mogga and Dr. Nancy Lesley. Phanidhar Mogga, Shreevidya Venkatraman, Urjitha Rajagopalan, Prashanth Rajagopalan, Prabhu Radhan, Kumaresan Maithrayie, Sivaraj Padmanabhan, Swamikannu Murugan, Archana Nagarajan, Chandrasekaran Venkataraman, Milly Mathew, and Georgi Abraham contributed and coordinated in drafting the final manuscript.
References
- Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-207.
- [Google Scholar]
- A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
- [Google Scholar]
- Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120-8.
- [Google Scholar]
- Cardiac endotheliitis and multisystem inflammatory syndrome after COVID-19. Ann Intern Med. 2020;173:1025-7.
- [Google Scholar]
- Interaction between systemic inflammation and renal tubular epithelial cells. Nephrol Dial Transplant. 2014;29:2004-11.
- [Google Scholar]
- Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31:1380-3.
- [Google Scholar]
- Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-92.
- [Google Scholar]
- The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: A systematic review and meta-analysis. Kidney Med. 2021;3:83-98. e1
- [Google Scholar]
- Risk factors for development of acute kidney injury in COVID-19 patients: A retrospective observational cohort study. Nephron. 2021;145:256-64.
- [Google Scholar]
- Acute kidney injury and mortality risk in older adults with COVID-19. J Nephrol. 2021;34:295-304.
- [Google Scholar]
- Acute kidney injury in hospitalized children with COVID19. J Trop Pediatr. 2021;67((2))
- [Google Scholar]
- Clinical profile and outcomes of COVID-19 patients with acute kidney injury: A tertiary centre experience from South India? Clin Exp Nephrol 2021:1-9. doi: 10.1007/s10157-021-02123-7
- [Google Scholar]
- Incidence, risk factors and outcome of COVID-19 associated AKI- A study from South India. J Assoc Physicians India. 2021;69:11-12.
- [Google Scholar]
- Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1) Crit Care. 2013;17:204.
- [Google Scholar]
- Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622-7.
- [Google Scholar]
- Indian Council of Medical Research. Clinical management protocol: COVID-19 [Table 1, p. 4, 5] Available online: https://www. mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf (2021)
- Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961-73.
- [Google Scholar]
- Risk, predictors, and outcomes of acute kidney injury in patients admitted to intensive care units in Egypt. Sci Rep. 2017;7:17163.
- [Google Scholar]
- Traditional medicines prescribed for prevention of COVID-19: Use with caution. Nephrology (Carlton). 2021;26:961-64.
- [Google Scholar]
- Acute kidney injury in patients with the coronavirus disease 2019: A multicenter study. Kidney Blood Press Res. 2020;45:612-22.
- [Google Scholar]
- Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J Nephrol. 2021;34:173-83.
- [Google Scholar]
- Acute kidney injury in patients with COVID-19: A retrospective cohort study from Switzerland? Swiss Med Wkly. 2021;151:w20482. doi: 10.4414/smw.2021.20482
- [Google Scholar]
- Urine abnormalities predict acute kidney injury in COVID-19 patients: An analysis of 110 cases in Chennai, South India. Diabetes Metab Syndr. 2021;15:187-91.
- [Google Scholar]
- Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209-18.
- [Google Scholar]
- Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis. 2020;27:365-76.
- [Google Scholar]
- Peritoneal dialysis for acute kidney injury during the COVID-19 pandemic in New York City. Kidney Int Rep. 2020;5:1532-4.
- [Google Scholar]
- First and second COVID-19 waves in Japan: A comparison of disease severity and characteristics. J Infect. 2021;82:84-123.
- [Google Scholar]
- First and second waves of coronavirus disease-19: A comparative study in hospitalized patients in Reus, Spain. medRxiv 2020 2020.12.10.20246959 doi: https://doi.org/10.1101/2020.12.10.20246959
- [Google Scholar]
- Clinical characteristics of the first and second COVID-19 waves in the Bronx, New York: A retrospective cohort study. Lancet Reg Health Am. 2021;3:100041. https://doi.org/10.1016/j.lana.2021.100041
- [Google Scholar]







