Translate this page into:
Cytomegalovirus and Leishmania donovani coinfection in a renal allograft recipient
Address for correspondence: Dr. Narayan Prasad, Department of Nephrology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow - 226 014, India. E-mail: narayan@sgpgi.ac.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Infection is a leading cause of death in renal allograft recipients. Apart from the immunosuppressive drugs, immunomodulatory viral infections also predispose the recipient to many opportunistic infections. Kala-azar in renal allograft recipients is infrequently reported even in endemic areas. In majority of cases, there was delay in diagnosis and treatment. We report a case of renal allograft recipient, where we faced a diagnostic dilemma because of coinfection of cytomegalovirus and visceral leishmaniasis (kala-azar). Kala-azar was successfully treated with amphotericin B. Kala-azar should always be kept as differential diagnosis in patients with pyrexia and cytopenia, even in the absence of splenomegaly in patients residing in an endemic zone.
Keywords
Cytomegalovirus infection
kala-azar
renal transplantation
Introduction
Infection is a leading cause of death in renal allograft recipients.[1] Apart from the immunosuppressive drugs, immunomodulatory viral infections also predispose the recipients to many opportunistic infections.[2–4] Visceral leishmaniasis (kala-azar) in renal allograft recipients is infrequently reported even in endemic areas. Leishmania infections in renal allograft recipients have been reported in about 35 cases, a negligible number if compared with large number of renal grafts.[5] We have reported two cases of visceral leishmaniasis.[67] In majority of cases including ours, there was delay in diagnosis and treatment. This may account for the 20 to 30% mortality described in renal transplant recipient.[35] Coexistence of different infections in renal transplantation leads to difficulties in diagnosis and treatment, and further increase in risk of morbidity and mortality.[7–9]
The coinfection of cytomegalovirus (CMV) with Leishmaniasis produces a diagnostic dilemma due to similarity of some clinical features and hematological parameters like fever, leucopenia, and thrombocytopenia. Hence, we report an unusual presentation of renal allograft recipient who had coinfection of CMV and visceral leishmaniasis. Leishmania donovani bodies were detected on bone marrow smear, and patient was successfully treated with amphotericin B.
Case Report
A 35-year-old man, resident of Bihar, was admitted to our hospital with 2 month history of high-grade fever, weakness, and myalgia. He had received a live-related renal transplantation in 1998 at our institute. He had basic disease of chronic interstitial nephritis secondary to obstructive uropathy and bilateral pelvic ureteric junction obstruction. Both recipient (R) and donor (D) were CMV IgG positive (D+ /R+ status). The recipient had an uneventful postoperative course and was discharged on triple immunosuppression with cyclosporine, azathioprine, and prednisolone. After one year, he developed steroid resistant acute cellular rejection which was treated with six doses of OKT. His graft function improved but did not touch baseline and serum creatinine remained high at 1.9 mg/dl. Following this event, he was doing well with stable renal functions and mild hypertension until the start of the present illness. His investigations during this period were hemoglobin, 12.5 g/dl; total leukocyte count, 5.6 × 103/μl; blood urea nitrogen, 38 mg/dl; and serum creatinine, 1.9 mg/dl.
His present illness started since February 2005, when he had high-grade fever (103°F) with generalized weakness and myalgia. On admission (April 2005), he was febrile (104°F) with tachycardia (102 beats per minute) and blood pressure of 136/90 mmHg.
Systemic examination was normal and there was no hepatomegaly or splenomegaly on per abdominal examination. His investigations at admission revealed hemoglobin, 8.4 g/dl (anemia); total leukocyte count, 0.8×103/μl (leucopenia); platelet count, 45×103/μl (thrombocytopenia); creatinine, 5.2 mg/dl; Na+ 132 mmol/l; K+, 4.6 mmol/l; total protein, 6.4 g/dl; and albumin, 4.1 g/dl. There was mild elevation of liver enzymes, alanine aminotransferase (68 U/l), and aspartate aminotransferase (82 U/l).
In view of fever, myalgia, leucopenia, thrombocytopenia, graft dysfunction, and elevated liver enzymes, provisional diagnosis of CMV disease was made. His CMV IgM was positive and CMV DNA was detected on polymerase chain reaction. He was started on intravenous ganciclovir, dose modified for his renal dysfunction. Azathioprine was stopped due to low total leukocyte count and elevated liver enzymes, and patient was continued on duel immunosuppression, prednisolone and cyclosporine. He was supported with granulocyte macrophage colony stimulating factor for severe leucopenia during this period. He had also developed epistaxis, for which he has been given platelet transfusion and epistaxis was stopped. He became afebrile after 7 days of ganciclovir therapy.
He was discharged after 14 days of intravenous ganciclovir in a stable condition on oral ganciclovir maintenance therapy. The discharge hemoglobin, was 9.6 g/dl; leukocyte count, 5.2×103/μl, platelet, 146×103/μl; and serum creatinine, 3.4 mg/dl. He remained afebrile for about a week, but again noticed low-grade fever (100°F), leucopenia (2.9×103/μl), thrombocytopenia (67×103/μl), and decline of graft function (serum creatinine, 5.6 mg/dl).
Ganciclovir was stopped in view of persistent leucopenia and thrombocytopenia. He was readmitted and CMV infection resistant to ganciclovir was considered. Bone marrow examination was done because of the relapse of fever, persistence of leucopenia and thrombocytopenia, and revealed evidence of amastigote form of Leishmania donovani [Figure 1], the causative organism of kala-azar. He was treated for 2 weeks with amphotericin-B at 1 mg/kg/day. He has tolerated the amphotericin B without any significant complication, except occasional mild fever with chill during infusion; became afebrile; and showed improvement in his renal functions (serum creatinine, 2.9 mg/dl) and total leukocyte count (6.8×103/μl) and platelet count (180×103/μl). There was no recurrence of leishmaniasis and graft function (serum creatinine, 2.9 mg/dl) remained stable.
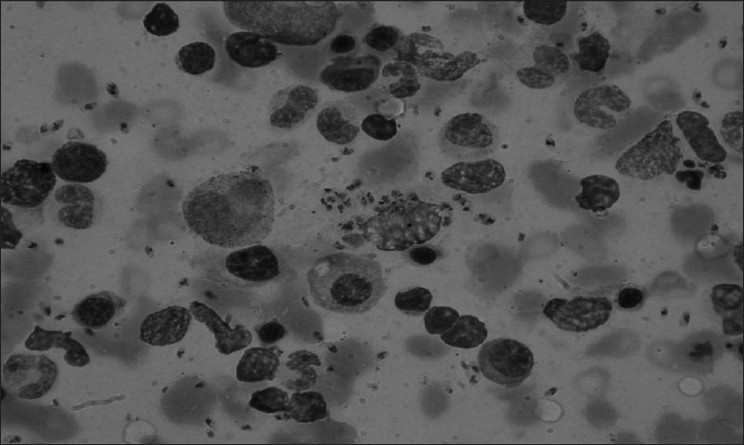
- Morphological examination of bone marrow aspirate showing Leishmania donovani bodies
Discussion
We report a renal transplant patient who had coinfection with CMV and Leishmania. The patient was first treated for CMV disease and later for kala-azar. This patient may have had coinfection at the time of presentation or may have developed sequential infection, first CMV followed by kala-azar. The patient was asymptomatic only for a brief period of 1 week after Ganciclovir therapy and there was recurrence of fever, leucopoenia, and thrombocytopenia despite maintenance therapy with oral ganciclovir. Therefore, we have suspected coinfection rather than sequential infection of CMV.
This patient belonged to an endemic region of kala-azar. But we did not suspect kala-azar at presentation, because there was no splenomegaly in this patient and CMV infection is more common than kala-azar in renal transplant recipients even in endemic regions.[5] The simulating clinical features of kala-azar and CMV disease developed diagnostic dilemma and delayed the diagnosis in this patient. The clinical diagnosis of kala-azar in the absence of splenomegaly is rare. Splenomegaly is reported in up to 100% cases in nontransplant patient.[10] Sabbatini et al.[5] have also reported splenomegaly in 86% of cases of kala-azar in solid organ allograft recipients. However, it may be absent in immunocompromised patient such as renal transplant patients, human immunodeficiency virus infection, and patient on long-term steroid therapy.[10]
Coexistence of different infections with similar clinical features and low index of suspicion often lead to delayed diagnosis in renal transplant recipients. This patient was treated for CMV infection first and he became afebrile, and leucopoenia and thrombocytopenia also improved. In this patient, the improved symptoms and cytopenia appear to be the effect of granulocyte macrophage colony stimulating factor and the treatment of CMV disease, but coexistence of Leishmania infection caused relapse of symptoms and development of pancytopenia.
CMV has immunomodulating and immunosuppressant effect, predisposing the recipient to other infections.[239] It is therefore possible that the CMV disease triggered the clinical presentation of this parasitic infection. However, CMV may also be part of superinfections reported in patients of kala-azar.[8] The immune response during the amastigote infection is related to cell-mediated immunity.[11] The eradication of amastigote form of Leishmania donovani is prevented by corticosteroids and cyclosporine. Corticosteroids block the macrophage activation and cyclosporine inhibits the lymphocyte activation cascade by acting mainly on CD4 T-helper cells and inhibits the production of cytokines and interferon-α. The impairment of cell-mediated immunity results into overt manifestations of kala-azar.[12]
Our patient had deterioration of graft function, which recovered spontaneously with treatment of kala-azar. Acute interstitial nephritis has been reported in patient with visceral leishmaniasis, and acute interstitial nephritis usually resolves with specific treatment of infection.[1314] The cause of graft dysfunction remains speculative in the absence of graft biopsy, but there was recovery of graft function only with treatment of infection. This suggests that kala-azar-induced acute interstitial nephritis may be responsible for his graft dysfunction.
Visceral leishmaniasis is endemic in southern Europe, tropical countries, and several parts of India. Kala-azar is potentially fatal if unrecognized and untreated in immunocompromised host. Latent infection can also progress to overt disease after immunosuppression.[1215] In a review of 29 renal transplant patients who developed kala-azar, seven were dead, usually as a consequence of other infection.[5] Our patient tolerated conventional amphotericin-B. Liposomal amphotericin B was not given due to high cost of therapy.
Pentavalent antimonials was not considered due to high toxicity[216] and resistant of this drug in this zone of kala-azar.[17] Our patient does not have evidence of any other superinfection and survived after treatment. This patient was followed up for more than a year and there was no recurrence of the disease. Relapse of kala-azar in transplant recipients has been reported in up to 31% cases after treatment with pentavalent antimonials.[2]
This case highlights the problem of pyrexia and cytopenias of unknown origin in a renal transplant patient living in an endemic zone for various tropical infections like kala-azar and its successful treatment with amphotericin-B. Coexistence of infections with similar clinical features like CMV and a low index of suspicion could delay diagnosis and treatment. Kala-azar should always be kept as differential diagnosis in patients with pyrexia and cytopenias, even in the absence of splenomegaly in a patient from endemic zones.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Infection in renal transplant recipient, Current approach to diagnosis, therapy and prevention. Am J Med. 1986;81:2-10.
- [Google Scholar]
- Visceral Leishmaniasis (Kala-azar) in solid organ transplants: case report and review. Transplantation. 1998;65:1401-4.
- [Google Scholar]
- Visceral Leishmaniasis (Kala-azar) in solid organ transplants: report of five cases and review. Clin Infect Dis. 1999;29:918-21.
- [Google Scholar]
- The independent role of Cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston center for liver transplantation CMVIG- study group. Cytogam, MedImmune,Inc. Maryland. Am J Med. 1997;103:106-13.
- [Google Scholar]
- Visceral leishmaniasis in renal transplant recipients. Is it still a challenge to the nephrologist? Transplantation. 2002;73:299-301.
- [Google Scholar]
- Visceral leishmaniasis: An unusual case of fever in a renal transplant recipient. Nephrol Dial transplant. 1991;6:736-8.
- [Google Scholar]
- Visceral leishmaniasis in a renal transplant recipient: diagnostic and therapeutic problems. Am J Nephrol. 1996;16:358-60.
- [Google Scholar]
- A renal transplant recipient with pulmonary tuberculosis and visceral leishmaniasis: Review of superimposed infections and therapy approaches. Clin Nephrol. 2003;60:289-94.
- [Google Scholar]
- Recurrent cytomegalovirus disease, visceral leishmaniasis, and legionella pneumonia after liver transplantation: a case report. Can J Anaeth. 2004;51:84-7.
- [Google Scholar]
- Leishmaniasis. In: Manson, ed. Manson's tropical disease (20th ed). London: WB Saunders; 1996. p. :1213-45.
- [Google Scholar]
- Visceral leishmaniasis: a rare cause of post-transplant fever and pancytopenia. J Assoc Physicians India. 2002;50:979-80.
- [Google Scholar]
- Therapy of visceral leishmaniasis in renal transplant recipients intolerant to pentavalent antimonials. Transplantation. 2000;70:800-1.
- [Google Scholar]







