Translate this page into:
Deceased donor organ transplantation: A single center experience
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Renal transplantation (RTx) is the best therapeutic modality for patient suffering from end-stage renal disease (ESRD). Deceased donor organ transplantation (DDOT) accounts for <4% of RTx in India. We report 4 years single centre experience on DDOT vis-à-vis patient/graft survival, graft function in terms of serum creatinine (SCr), rejection episodes, and delayed graft function in 160 DDOT. Between January 2006 to December 2009, 160 RTx from 89 donors were performed, of which 25.2% were expanded criteria donors. Majority of the donors were brain dead due to road traffic/cerebrovascular accidents. The commonest recipient diseases leading to ESRD were chronic glomerulonephritis (49%), diabetes mellitus (10%), and benign nephrosclerosis (10%). Mean recipient/donor age was 35.6±14.68 and 44.03±18.19 years. Mean dialysis duration pretransplantation was 15.37±2.82 months. Mean cold ischemia time was 5.56±2.04 hours. All recipients received single dose rabbit-anti-thymocyte globulin induction and steroids, mycophenolate mofetil/calcinueurin inhibitor for maintenance of immunosuppression. Delayed graft function was observed in 30.6% patients and 14% had biopsy proven acute rejection. Over mean follow-up of 2.35±1.24 years, patient and graft survival rates were 77.5% and 89.3% with mean SCr of 1.40±0.36 mg/dl. DDOT has acceptable graft/patient survival over 4 years follow-up and should be encouraged in view of organ shortage.
Keywords
Deceased donor
graft survival
patient survival
renal transplantation
Introduction
Renal transplantation (RTx) is best therapeutic modality for end-stage renal disease (ESRD).[1] Compared with dialysis, a transplant leads to a longer life,[2] enhances quality of life,[3] and is cost-effective for the health care system.[34] In India, 175,000 new patients develop ESRD annually, and <10% are able to gain access to renal replacement therapy. The rate of renal transplantations performed yearly in India translates to 3.25 per million population; the deceased-donation rate is 0.08 per million population per year.[56] This discrepancy between the number of waiting patients and transplantations performed can be reduced by developing deceased donor organ transplantation (DDOT) program. The reasons for such a low rate are many, ranging from lack of awareness to socioeconomic reasons.[7] Apart from the medical issues, legal, social, and ethical issues are the key factors in obtaining consent from the relatives of potential deceased donors.[8] We present our experience of DDOT over last 4 years.
Materials and Methods
This was a retrospective study of 160 DDOT carried out in our institute from January 2006 to December 2009. Both kidneys were procured from all donors and preserved in HTK solution. Demographics and post-transplant follow up including investigations, immunosuppression requirement, rejection episodes, and survival were evaluated. Patient survival was defined as time from transplantation to death. Graft survival was defined as time from transplant to requirement for hemodialysis.
Immunosuppressive regimen
All patients received induction with rabbit-anti-thymocyte globulin (r-ATG) (1.5 mg/ kg), and methylprednisolone (MP) 500 mg intravenously and MP was continued for 3 days postoperatively. Maintenance of immunosuppression consisted of prednisolone (30 mg/day tapered to 10 mg/day at 3 months post-transplant and continued thereafter), mycophenolate mofetil (MMF) (2 g/day), and calcineurin inhibitors (CNI) [cyclosporine {CsA} (5 mg/kgBW/day or tacrolimus, 0.08 mg/kgBW/day)]. Doses of CNI/sirolimus were adjusted as per trough levels. Doses of CNI were adjusted as per trough levels (C0) by HPLC method in initial 2–3 months thereafter it was done in event of graft dysfunction due to economic constraints.Cyclosporine dosing was adjusted to achieve target C0 concentrations of 200–300 ng/ml during the first 2–3 months after transplantation, 100–250 ng/ml up to 6 months after transplantation, and ~100 ng/ml thereafter. Tacrolimus dosing was adjusted to achieve target C0 concentrations of 10–15 ng/ml during the first 2–3 months after transplantation, and 4–8 ng/ml thereafter.
Sirolimus was used in event of CNI toxicity/ intolerance. All patients received prophylaxis against cytomegalovirus (CMV) infection (gancyclovir 1 g thrice a day×3month), fungal infections (fluconazole 100 mg once a day×6 months), and pneumocystis carinii pneumonia (trimethoprim/sulfamethaxazole (TMP/SMX 160/800 mg) once a day×9 months).
Post-transplant follow-up
All patients were followed at weekly intervals for the first 3 months, fortnightly for the next 3 months, monthly for the next 6 months, and 3 monthly intervals thereafter. On every visit, renal and liver function status was monitored; complete blood counts and ultrasound Doppler studies were performed.
Diagnosis and treatment of rejection
Recipients underwent renal graft biopsy for clinical suspicion of acute rejection based on a decline in renal function. An acute rejection episode diagnosed by an allograft biopsy as per the modified Banff classification,[910] was treated with standard anti-rejection therapy (ART). T-cell rejections were treated with MP 500 mg×3 doses±r-ATG 1.5 mg/kg single dose. B-cell rejections were treated with MP 500 mg × 3 doses±plasmapharesis (40 ml/kg per session×4–8 sessions)+IVIG 5 g/day×5–10 doses±rituximab 375 mg/m2 BSA single dose.
Results
Out of 1109 RTxs performed, 160 (14.42%) were DDOT, out of which 68.8% (n=110) were males and 31.2% (n=50) were females with mean age, 35.6±14.68 years. Mean duration of hemodialysis pretransplantation was 15.37±2.82 months. The underlying diseases for development of ESRD were chronic glomerulonephritis (CGN) (n=79), diabetes mellitus (n=16), benign nephrosclerosis (n=16), autosomal dominant polycystic kidney disease (n=9), focal and segmental glomerulosclerosis (n=3), IgA nephropathy (n=4), obstructive uropathy (n=8), tubulointerstitial nephritis (n=7) , lupus nephritis (n=6), single unit kidney with CGN (n=5), MPGN (n=2), chronic pyelonepritis(n=2), and alport syndrome (n=3). Kidneys were retrieved from 89 donors, including 14.3% non-heart-beating donors and 25.2% expanded criteria donors (ECD). Ten percent (n=16) patients received dual kidneys and two were discarded. The mean donor age was 44.03±18.19 years. Main cause of brain death was road traffic/cerebrovascular accidents. Out of 89 donors, 24.7% (n=22) were from Ahmedabad, 55.1% (n=49) from Surat (about 250 km away), 16.9% (n=15) from Rajkot (about 200 km away), 2.2% (n=2) from Bhuj (about 600 km away), and 1.1% (n=1) was from Bhavnagar (about 250 km away). Mean cold ischaemia time was 5.56±2.04 h and a total of 30.6% (n=49) patients developed delayed graft function.
Patient survival was 79.58%, 76.7%, 74.8%, and graft survival was 92.4%, 87.9%%, and 87.9% at 1, 2 and 3 years respectively [Figure 1]. Mean SCr (mg/dl) at 1 year, 2 years and 3 years was 1.34±0.34, 1.43±0.41, and 1.40±0.36, respectively.
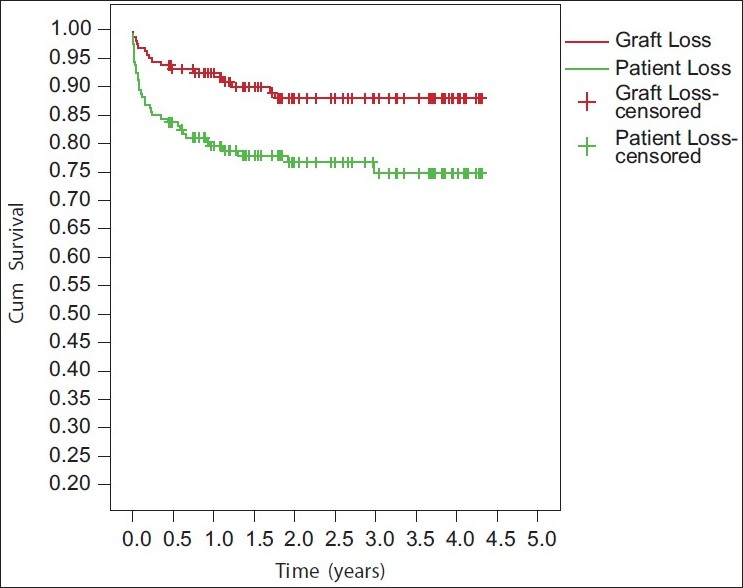
- Kaplan curve showing patient and graft survival
Total of 36 (22.5%) patients died, 16 due to gram-negative sepsis, 4 due to fungal infection, 4 due to CMV disease, 3 due to staphylococcal pneumonia with acute respiratory distress, 4 due to acute myocardial infarction, and 5 due to cerebrovascular stroke.
All patients received triple immunosuppression, 60% (n=96) received CsA, 31.9% (n=51) received tacrolimus, and 8.22% (n=13) received Sirolimus-based regimens.
14% (10% in 1st year and 4% in 2nd year) had biopsy proven acute rejection (n=22). Acute B-cell mediated rejections were noted in 3.8% (n=6), acute T-cell mediated rejections in 2.5% (n=4), combined acute T- and B-cell mediated rejections in 3.8% (n=6), acute borderline T+B-cell mediated rejections were noted in 3.8% (n=6), and chronic T-cell mediated rejection was noted in 0.63% (n=1) patients. Majority of the rejections were observed in first year. Nineteen patients responded to anti-rejection therapy.
Discussion
DDOT in India is low despite tremendous potential. Following steps which are prerequisite for successful DDOT program in our set-up have been taken by our institute.[7811–15]
-
Increased public awareness on the need for deceased donor organs like public education campaigns on brain death especially on world kidney day.
-
Encouraging participation of surgeons in general hospitals in identification of potential donors.
-
Expediting in passing legislation to procure deceased donor organs.
-
Reduction of cost of transplantation (since the financial burden is borne by individuals with state support).
-
Early brain death identification and certification by neurosurgeons, recognizing potential donors.
-
Requesting for organ donation and obtaining consent for organ donation.
-
Establishment of a committed and integrated rapid response team capable of transporting potential donor to hospitals in a timely manner.
-
Improvement in transportation, communication networks, and ambulance services, successful organ retrieval.
-
Efficient and trained transplant co-coordinator.
-
Adequate hospital infrastructure and support logistics along with intensivists with adequate intensive care facilities and fully qualified, trained medical and paramedical staff.
-
Inclusion of expanded criteria donors (ECD).
-
Positive steps have been taken by the central and state governments, and few non-government (NGO)/nonprofit organizations in the country.
DDOT with elderly donors or donors with suboptimal donation criteria (“marginal” or ECD) are increasing in number. In the United States, 15%–20% of donors were ECD in 2002.[12] In Europe, according to Eurotransplant data in 2007, 20% of donors were ≥65 years old.[12] In India, where DDOT account for around 4% of total transplants, discarding the marginal kidneys would hamper the program. In this study, ECD comprised 25.2% of DDOT. In the circumstances of organ shortage, DDOT with expanded criteria donor is a feasible option.
Individual centers in the India have reported their outcomes: In a study by Mani, 1 year and 4 year graft survivals of 88 DDOT in Chennai were 72% and 63%, respectively, and patient survival was hardly different from graft survival. The inability of most of the patients to afford monoclonal antibodies for immunosuppression might be the reason for inferior 1-year results in this study.[13] Five-year patient and graft survivals of 68 DDOT in Chennai were 61.7% and 58.8%, respectively, with biopsy proven acute rejection (BPAR), 26.4%, delayed graft function (DGF) in 50% and cold ischemia time (CIT) was 5.6±3.2 h, respectively. Short-term graft survival was reduced because of a high 1-year post-transplantation mortality, with most of these deaths caused by sepsis or multiple organ failure. Risk factors for early graft loss were retransplantation, longer cold ischemia time, and acute rejection episode.[14]
The 1-year allograft and patient survivals, of 100 DDOT from four major centers in Chennai, were 82% and 86%, respectively, with 2-year allograft and patient survivals of 74% and 80%, respectively.[15] In a study by Feroz et al., from our centre, 1-year patient and graft survival rate for 38 DDOT were 90% and 85%, respectively, with BPAR in 17%, DGF in 68% and CIT of 6.9±3.8 h, respectively.[7] In Indian, graft survival rates in good centers are 80% in cadaver donor transplants.[5] In our study, over mean follow-up of 2.35±1.24 years, patient and graft survival rates were 77.5% and 89.3% respectively with high 1-year post-transplantation mortality, with most of these deaths caused by sepsis. It is possible that triple immunosuppressive regimens with ATG induction, unhygienic living conditions, delayed presentation and diagnosis, tropical climate, limited availability, and the expense of diagnostic tools and economic constraints for treatment in majority of patients, may have contributed to high infection rate, similar to experience by other studies.[1316–18] Infection, long duration of HD before transplant, ECD, increased DGF, socioeconomic factors pervasively influenced access to health care, may have contributed to high 1-year post-transplantation mortality, with most of these deaths caused by sepsis.[19–21] Recipients of renal allograft in developing countries may be more prone to infections, which are the most common cause of post-transplant mortality.[22]
The shortcoming of this analysis included its retrospective single centre evaluation and variable immunosuppressive regimens
Conclusion
In the circumstances of organ shortage DDOT have a potential to expand the donor pool and shorten the waiting lists for RTx. Graft function along with patient and graft survival rates are also acceptable.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-30.
- [Google Scholar]
- The quality of life of patients with end-stage renal disease. N Engl J Med. 1985;312:553-9.
- [Google Scholar]
- A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50:235-42.
- [Google Scholar]
- Resource settings have a major influence on the outcome of maintenance hemodialysis patients in South India. Hemodial Int. 2010;14:211-7.
- [Google Scholar]
- Five decades of Indian nephrology: A personal journey. Am J Kidney Dis. 2009;54:753-63.
- [Google Scholar]
- Cadaveric renal transplantation: Our experience at the Institute of Kidney Diseases and Research Centre, Institute of Transplantation Sciences, Ahmedabad. Transplant Proc. 2007;39:721-2.
- [Google Scholar]
- Improving cadaveric organ donation rates in kidney and liver transplantation in Asia. Transplant Proc. 2004;36:1873-5.
- [Google Scholar]
- Antibody-mediated rejection criteria -An addition to the Banff’97 classification of renal allograft rejection. Am J Transplant. 2003;3:708-14.
- [Google Scholar]
- Banff’05 Meeting Report: Differential Diagnosis of Chronic Allograft Injury and Elimination of Chronic Allograft Nephropathy (‘CAN’) Am J Transplant. 2007;7:518-26.
- [Google Scholar]
- Deceased donor organ transplantation with expanded criteria donors: A single-center experience from India. Transplant Proc. 2010;42:171-4.
- [Google Scholar]
- Review article, development of cadaver renal transplantation in India. Nephrology. 2002;7:177-82.
- [Google Scholar]
- Cadaveric renal transplantation: The Chennai experience. Transplant Proc. 2008;40:1104-7.
- [Google Scholar]
- Cadaver organ donation and transplantation-an Indian perspective. Transplant Proc. 2003;35:15-7.
- [Google Scholar]
- Infections in dialysis and transplant patients in tropical countries. Kidney Int. 2000;57:S85-93.
- [Google Scholar]
- Duration of end-stage renal disease and kidney transplant outcome. Nephrol Dial Transplant. 2005;20:167-75.
- [Google Scholar]
- Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66:1697-701.
- [Google Scholar]
- The alphabet soup of kidney transplantation: SCD, DCD, ECD—fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol. 2009;4:1827-31.
- [Google Scholar]
- Results in 158 consecutive cadaveric renal transplantations. Transplant Proc. 2005;37:2965-6.
- [Google Scholar]







