Translate this page into:
Deceased donor renal transplantation: A single center experience
Address for correspondence: Dr. T. Dineshkumar, Department of Nephrology, Madras Medical College and Rajiv Gandhi Government General Hospital, Chennai, Tamil Nadu, India. E-mail: dinesh.thaniga@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Deceased donor renal transplantation (DDRT) constitutes less than 5% of all kidney transplantats in India. A retrospective analysis of 173 deceased donor renal transplants performed in a public funded government hospital was done. Mean age of the recipients was 36 years (male:female ratio 2.4:1), and that of the donors was 32.3 years (male:female ratio 6:1). The cold ischemic time was 340 ± 170 minutes. Mean follow-up period was 36 months. Forty one patients died, 75% of them in the first post – transplant year. Sepsis and cardiovascular disease were the most common causes of death. Twenty two percent had acute rejection. There was no significant difference in the incidence in the rate of acute rejection, bacterial, fungal infections and death rate between the cohorts of induction and non induction immunosuppression. The patient and death censored graft survival at 1 year were 80 and 82.6% and at 5 years were 76 and 80% respectively.
Keywords
Deceased donor
graft survival
immediate graft function
patient survival
Introduction
Deceased donor renal transplantation (DDRT) is still infrequent in India, constituting less than 5% of the total renal transplants of about 3500 per year.[1] Only 35 out of 200 approved renal transplant centers perform DDRT regularly.[2] Hence, there is paucity of Indian data on DDRT. Tamil Nadu has an active DDRT program, with donation rate of is 0.3 per million population against the national rate of only 0.08 per million.[34] The state government of Tamil Nadu has established a robust and effective DDRT model. The state government, public and private hospitals and non-governmental voluntary organizations work in unison accomplishing their respective defined responsibilities. Transplant Authority of Tamil Nadu is an autonomous government body which governs all the aspects of the programme viz. brain death certification, organ retrieval, and allocation. This study was done to measure the outcomes of DDRT in a public-funded tertiary care hospital.
Materials and Methods
A retrospective analysis of 173 deceased donor renal transplants performed between January 1995 and September 2015 was done. All the transplants were blood group compatible. Data analysis included age, sex, native kidney disease, cold ischemia time, delayed graft function (DGF), dialysis requirement, rejection episodes, infective episodes, and new onset diabetes after transplant (NODAT). Donor characteristics viz. age, sex and age difference between recipient and donor were studied. Human leukocyte antigen (HLA) matching could not be done due to logistic reasons. Crossmatch test was carried out by complement-dependent microlymphocytotoxicity test.
Immunosuppressive regimen
Till 2009, all the patients received steroids, cyclosporine, and azathioprine. From 2010, the immunosuppressive regimen consisted of steroids, tacrolimus, and mycophenolate mofetil. Induction agents were given from 2012. Seventy-four patients had received induction agents. (anti-thymocyte globulin 57, basiliximab 17). The induction agent protocol was single dose of anti-thymocyte globulin at the dose of 1.5 mg/kg and two doses of basiliximab on day 1 and day 4. Primary cytomegalovirus prophylaxis was given for those who received induction agents. The acute cellular rejection was treated with 3 doses of intravenous methylprednisolone (500 mg each), and antibody-mediated rejection was treated with plasmapheresis (1 plasma volume removal per session over 4–8 sessions) and intravenous immunoglobulin (100 mg/kg/day over 5–10 doses).
Outcome definitions
Immediate graft function (IGF) was defined as non-requirement of dialysis after transplantation; delayed graft function (DGF) as need for dialytic support within a week of transplant, primary nonfunction (PNF) as patients whose graft never functioned.
Statistical methods
Patient data were entered on an Excel spreadsheet and imported to the MedCalc statistical program for analysis. (Medcalc software byba version 16.1USA) Data are presented as mean ± standard deviation unless otherwise specified. Cox proportional hazard univariate analysis was done to analyze the variables for graft and patient survival. The confidence interval was 95%. Continuous variables were studied using one way ANOVA and categorical analysis were done using Chi-square test.
P < 0.05 was used for statistical significance. Kaplan–Meier and Cox regression log ranks were used for survival analysis.
Results
One hundred and seventy-three patients underwent DDRT till September 2015. Mean follow-up period was 36 months (range 2–132 months). Amongst donors, there were 148 males and 25 females (M:F ratio: 6:1), with a mean age of 34 ± 13 years. There were 20 (11.5%) extended criteria donors (ECDs). The mean cold ischemic time was 340 ± 170 min. Recipients were stratified into three groups based on graft function: IGF (n = 63), DGF (n = 84), PNF (n = 26).
Graft function
Twenty-six patients had PNF. Multiple variables were studied in both IGF and DGF groups applying one-way ANOVA analysis [Table 1]. There was no statistically significant difference in donor age, recipient age, the age difference between donor and recipients and recipient gender between IGF (n = 63) and DGF (n = 84) groups. Mean cold ischemic time was shorter in patients with IGF group than in DGF group (262 vs. 382 min, P = 0.008). Number of patients with normal graft function at 12 and 24 months was significantly higher in the IGF group as compared to DGF group. (P = 0.034, and 0.040 respectively). The incidence of acute rejection was 21.8% (n = 37). Out of three patients who had antibody mediated rejection, two returned to dialysis, and one expired.

There was no statistically significant difference between IGF (n = 9/63) and DGF (13/84) groups in the incidence of acute rejection (P = 0.698). There was no difference in the incidence of NODAT and infection episodes due to cytomegalovirus, fungi, and tuberculosis among the two groups. At mean follow-up of 36 months, none of the patients in the IGF group had graft loss, while four patients in DGF group returned to dialysis (P = 0.024. Number of deaths in the IGF and DGF groups was 6 and 11, respectively (P = 0.04). Table 2 enumerates the cause of early and late deaths. Graft outcomes in patients who received induction agents were described in Table 3. The cold ischemic time, and incidence of DGF were higher in induction group when compared with no induction, mean cold ischemia time (CIT) and DGF was 380 min and 67% versus 246, 24%, (P value 0.0001, 0.0004) respectively between the induction and noninduction groups. All patients with DGF who remained dialysis dependent at 2 weeks underwent biopsy. Forty-eight subjects underwent graft biopsy. 35 (72%) showed acute tubular injury; eight patients had acute rejection, and five were found to have calcineurin inhibitor toxicity.


Patient and graft survival
The patient survival was 80.34% at 1 year, 79.7% at 2 years, 78% at 3 years, and 76% at 5 years. The graft survival was 82.6% at 1 year, 82% at 2 years, 81.5% at 3 years, and 80% at 5 years when death with functioning graft (DWFG) was censored. During follow-up, 41 patients died (23.6%); twenty patients with PNF of graft and remaining (n = 21) with functioning grafts [Table 2].
One year patient survival was 80.34% implying a high mortality in the first posttransplant year. Most of the deaths occurred during the first 3 months after transplant (45% in the 1st month [n = 18], 30% in one to 3 months [n = 13]). The causes of death in patients with nonfunctioning graft were sepsis (n = 7), severe acidosis (n = 6) and vascular complications (n = 6). In those who died with functioning graft (DWFG), sepsis was the most common cause (n = 14, [75%]) and malignancy (n = 1).
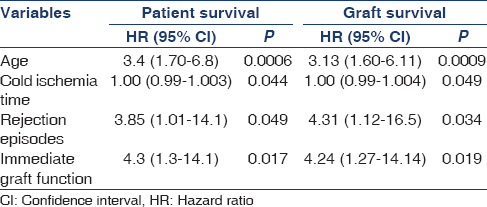
The median survivals for patients with IGF and DGF were 72 months and 34 months respectively [Figure 1]. Factors predicting patient and graft survival were analyzed by applying Cox regression analysis [Table 4 and Figure 2]. Age of the recipient, cold ischemic time, rejection episodes, and IGF after transplant were found to be independent predictors of patient and graft survival. Increasing age of the recipient was associated with high mortality.
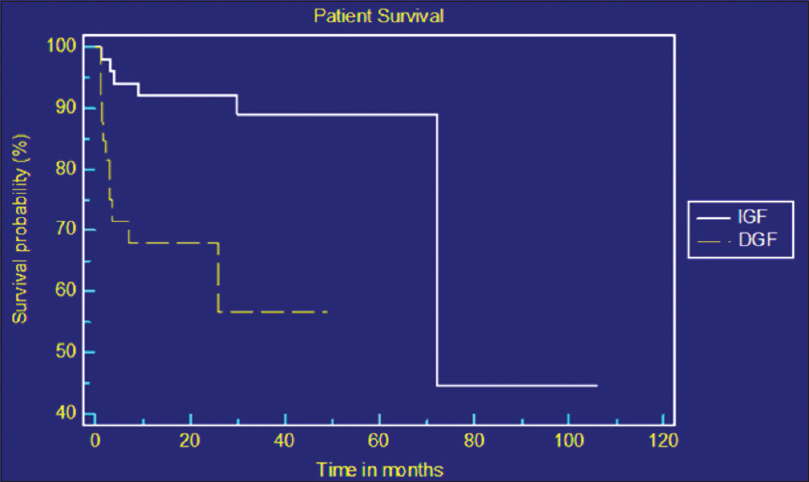
- Kaplan–Meier curve showing patient survival between immediate graft function and delayed graft function groups
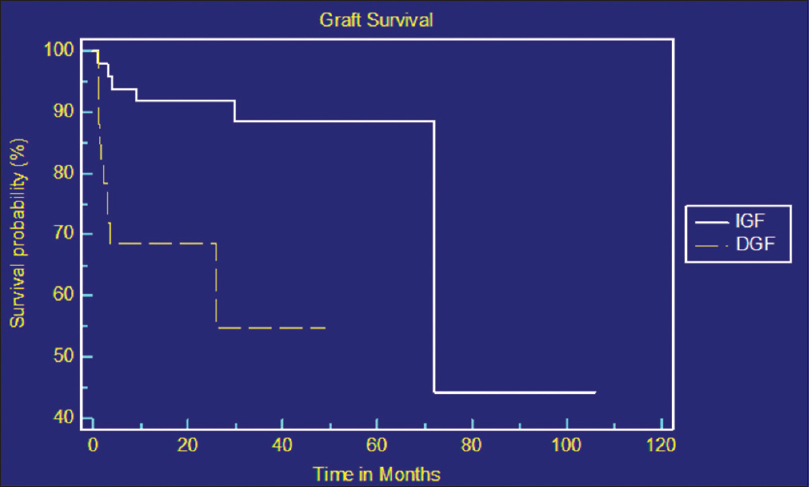
- Kaplan–Meier curve showing graft survival between immediate graft function and delayed graft function groups
Discussion
With the emergence of small and nuclear family pattern and the rising prevalence of hypertension and diabetes in India, there has been a significant decline in availability of suitable related donors. This pressing scenario has made “deceased donor transplant program” a compelling need of the hour.
In our study, 14.4% of donors and 28.9% of recipients were females. This is in contrast to the gender disparity noted in living related donor (LRD) transplant scenario in India in which majority of the donors are women while majority of the recipients are men. About 78% donors in our living related renal transplant program were females (unpublished data from Madras medical college). Bal et al. have highlighted gender disparity in a series of LRD renal transplants wherein 66% of donors were females and only 9.2% of recipients were females.[5]
In our study PNF contributed for high mortality in the 1st year after transplant. Majority of the PNF were due to graft vessel related complications and severe perioperative metabolic acidosis. The incidence of PNF has been declining during the previous 2 years.
The incidence of DGF was 48.5%. Prolonged CIT was found to be a risk factor for DGF. DGF had significant impact on graft function at 12 and 24 months and correlated with patient survival as well. This finding is in concordance with studies by Boom et al.[6] Helfer et al.[7] and Cheung et al.[8] The impact of DGF on long term patient and graft survival is controversial. Some studies reports DGF as an independent risk factor for poor graft survival while others report no negative impact on survival. Shoskes and Cecka have reported DGF significantly reduces graft half-life from 9.2 to 6.2 years.[9] Poor graft survival in DGF is attributed to repeated subclinical rejection and subsequent development of chronic allograft nephropathy. In our study, patients with DGF had significant number of DWFGs when compared to patients with IGF. This finding was consistent with the study by Tapiawala et al. who reported DGF was independent and significant risk factor for DWFG.[10]
Similar incidence of high mortality in 1st year after transplant is also reported by Patel et al.[11]
In a study from western India comprising 293 deceased donor transplants, incidence of DGF was 29%, acute rejection 22%, CIT 5.36 h, 1 and 5 years patient survival 81.7% and 77.5% and graft survival 92.6% and 88.3% respectively.[12] In our study, 48.5% had DGF, 21.8% had acute rejection, mean CIT was 5.66 h, 1 and 5 years patient survival 80.2% and 76.3% and graft survival 82.6% and 80%, respectively. When compared to other Indian studies,[131415] the incidence of DGF was higher in our study despite having shorter mean cold ischemic time [Table 5].
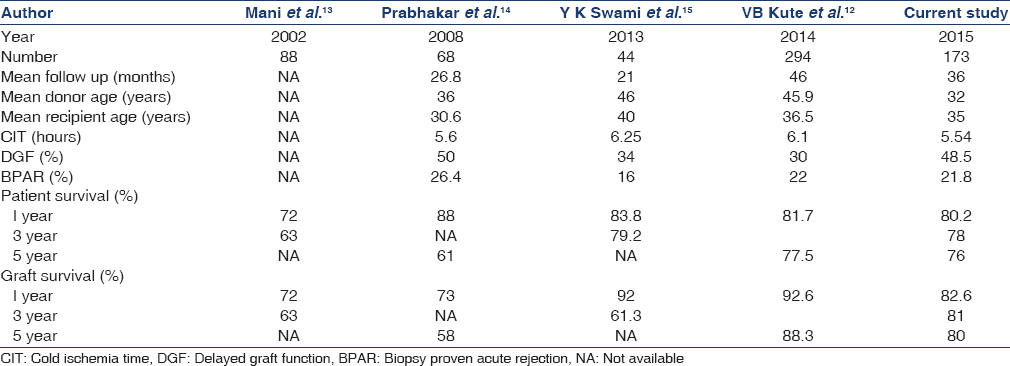
Increased cold ischemic time and incidence of DGF in induction group can be explained by following confounding factors, In the preinduction era, all the DDRT were done with 'in house' deceased donors within our center and kidneys alone were harvested which obviously had shorter CIT and less DGF On the contrary, in the induction era, most of our DDRT were from outside center and multiple organs were harvested per donor viz lungs, heart and liver, which resulted prolonged CIT and more DGF. But there is no difference in incidence of acute rejection, sepsis and death rate between the groups.
There was no gender difference in patient and graft survival, contrary to previous reports that female transplant recipients had better survival than male recipients.[16]
This study has limitations inherent for a retrospective study. Recipients of ECD organs were not studied as a separate cohort in view of small number, HLA matching could not be done due to logistic reasons, and the impact of donor renal dysfunction and potential donor sepsis on the graft outcome could not be analyzed.
Despite these limitations, DDRT model of Tamilnadu has proved that DDRT program catering to underprivileged patients is feasible in a government funded hospital and has evinced interest among other Indian states to emulate the program.[1718]
Conclusion
The single most important factor determining graft and patient survival in our study was the attainment of graft function immediately after transplant. The incidence of DGF was 48.5%, and acute rejection was 21.8% The patient and death-censored graft survival at 1 year were 80 and 82.6 and at 5 years was 76 and 80%, respectively. Use of induction agents does not show any significant difference in the incidence of acute rejection, sepsis and death.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the immense contribution of the departments of Urology, Anaesthesia, Neurosurgery and Immunology at Madras Medical College and Professor J Amalorpavanathan Member Secretary, Transplant Authority of Tamil Nadu.
References
- The incidence of end-stage renal disease in India: A population-based study. Kidney Int. 2006;70:2131-3.
- [Google Scholar]
- Five decades of Indian nephrology: A personal journey. Am J Kidney Dis. 2009;54:753-63.
- [Google Scholar]
- Tamil Nadu Cadaver Transplant Program. Waitlist Statistics for Kidney. Available from: http://www.tnos.org/Waitlist-details.asp
- Gender bias in renal transplantation: Are women alone donating kidneys in India? Transplant Proc. 2007;39:2961-3.
- [Google Scholar]
- Delayed graft function influences renal function, but not survival. Kidney Int. 2000;58:859-66.
- [Google Scholar]
- Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation in a Brazilian center. Transplant Proc. 2014;46:1727-9.
- [Google Scholar]
- Impact of delayed graft function on renal function and graft survival in deceased kidney transplantation. Hong Kong Med J. 2010;16:378-82.
- [Google Scholar]
- Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66:1697-701.
- [Google Scholar]
- Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21:153-61.
- [Google Scholar]
- Outcome of deceased donor renal transplantation – A single-center experience from developing country. Saudi J Kidney Dis Transpl. 2013;24:403-7.
- [Google Scholar]
- Outcome of renal transplantation from deceased donors: Experience from developing country. Ren Fail. 2014;36:1215-20.
- [Google Scholar]
- Review article, development of cadaver renal transplantation in India. Nephrology. 2002;7:177-82.
- [Google Scholar]
- Cadaveric renal transplantation: The Chennai experience. Transplant Proc. 2008;40:1104-7.
- [Google Scholar]
- Deceased donor renal transplantation at army hospital research and referral: Our experience. Indian J Urol. 2013;29:105-9.
- [Google Scholar]
- Early graft function and patient survival following cadaveric renal transplantation. Kidney Int. 1999;55:692-9.
- [Google Scholar]
- How deceased donor transplantation is impacting a decline in commercial transplantation-the Tamil Nadu experience. Transplantation. 2012;93:757-60.
- [Google Scholar]







