Translate this page into:
Early corticosteroid withdrawal regimen in a living donor kidney transplantation program
Address for correspondence: Dr. Shyam Bihari Bansal, Department of Nephrology, Medanta Kidney and Urology Institute, Medanta Medicity, Gurgaon - 122 018, Haryana, India. E-mail: shyambansal1974@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Steroids have been the essential component of transplant immunosuppression. Recently, with availability of better immunosuppressive agents, many centers have started steroid free transplant with good success rates. We analyzed the outcomes of early corticosteroid withdrawal (CSW) protocol in our living donor kidney transplant programme. We included 73 patients on CSW protocol on basiliximab + tacrolimus and mycophenolate mofetil and compared them with 67 recipients on similar regimen with corticosteroids (CSs). CSW group received prednisolone 40 mg on day 1, which was stopped on day 5. Outcomes were evaluated in terms of acute rejection (AR), infections, new onset diabetes after transplant (NODAT), renal function and graft or patient loss. In CSW group, 15/73 (20.5%) patients developed AR, when compared to 5/67 (7.5%) in CS group, (P = 0.02). Biopsy proven acute rejection was seen in 12/72 (16.6%) in CSW group and 5/67 (7.5%) in CS (P = 0.1). One patient in CSW group developed antibody mediated rejection. NODAT was similar (9% in CS vs. 3.7% in CSW, P = 0.09), but infections were higher in CSW group (20.5% vs. 7.5%, P = 0.02). Mean serum creatinine was similar at 6 months (1.24 ± 0.6 in CS and 1.25 ± 0.3 in CSW, P = 0.9). Graft survival was 100% and 97% (P = 0.1) and patient survival was 98.6% and 98.5% (P = 0.9) in CSW and CS groups. Early corticosteroid withdrawal with basiliximab induction was associated with increased risk of AR but did not have any effect on short term graft and pateint survival.
Keywords
Steroid free
kidney transplantation
living donors
Introduction
Corticosteroids (CSs) have been an integral part of immunosuppressive protocols in kidney transplantation since the beginning of transplantation. However, in recent years there is an increasing trend of using complete steroid free (CSF) or early corticosteroid withdrawal (CSW) protocol in many centers around the world. This is to avoid side-effects related to prolonged steroid usage such as new onset diabetes after transplant (NODAT), osteoporosis, hyperlipidemia, hypertension and increased risk of cardiovascular disease.[12] Prolonged use of even low dose steroids (<7.5 mg prednisolone) has been associated with adverse effects such as acne, weight gain, cataract and skin bruising.[34] However withdrawal of steroids in earlier studies was associated with an increase in incidence of chronic rejections (CR) and late graft loss when steroids were withdrawn after 3-6 months.[567] Recently with the availability of better immunosuppressive drugs such as tacrolimus (TAC), sirolimus, mycophenolate mofetil (MMF) and induction with polyclonal or monoclonal antibodies, many centers have successfully performed CSF/early CSW transplantation.[891011] Unlike before, when steroid withdrawal was attempted late, these centers have practiced either early CSW, i.e. within the first week of transplant or not used steroids at all. Short-term results of these studies are good with either equal or slightly increased risk of acute rejections (ARs), without any effect on graft and patient survival. Some studies have shown benefit in term of NODAT, lipid profile, use of antihypertensive drugs and other adverse effects.[111213]
In India, kidney transplantation is mainly done from living donors and there is no report of using CSF or early CSW protocol in these patients. We undertook this study to see the efficacy and safety of early CSW on day 5 in recipients of the first living donor kidney transplant on a regimen of TAC, MMF with basiliximab induction and compared them with consecutively transplanted patients on similar regimen along with CS.
Materials and Methods
Patients
The study was conducted at Medanta-The Medicity, Gurgaon, India. Seventy three patients were enrolled between February 2010 and December 2011. Consenting patients, aged more than 10 years, with chronic kidney disease (CKD) stage V undergoing first kidney transplant with a living donor were included in the study. Patients were included if they received kidneys from HLA identical, one haplotype match or spousal donors. Patients were excluded if they received a kidney from a deceased donor, re-transplant, multi-organ transplant, induction with thymoglobulin, historic cross match positive and previous history of using steroids. Patients were also excluded if patient or donor had HIV positivity; significant liver disease; malignancy or a history of malignancy.
Study design
This was a retrospective analysis, in which safety and efficacy of early CSW at day 5 with a regimen of basiliximab induction along with TAC and mycophenolate was studied and compared with 67 consecutively transplanted patients with similar immunosuppression along with CS. These patients were recruited between February 2010 and December 2011 and followed-up at least for a period of 6 months. Before enrolment, all the patients who fulfilled the inclusion criteria were explained in detail about benefits and risks of early steroid withdrawal including higher probability of rejection. Patients who chose to receive CSW after a thorough counseling were included in the study after a written and informed consent. The study was carried out in accordance with the principles of the declaration of Helsinki.
Immunosuppression
All patients were initiated on oral TAC, 0.1 mg/kg/day in two divided doses 1 day prior to surgery. Patients received two doses of TAC on day-1 and one dose in the morning before going for surgery. TAC whole blood trough levels were kept between 8-12 ng/ml in first 3 months, 6-8 ng/ml between 3-6 months and 3-6 ng/ml thereafter. TAC levels were done by chemiluminiscense (Abbott)-method at least twice in 1st week, once a week in 1st month, once in 15 days for next 2 months and monthly thereafter. In addition, TAC levels were done whenever there was graft dysfunction or felt by the physician for managing the patient. MMF or MMF sodium (MMF Na) was started at a dose of 1000 or 720 mg respectively twice daily orally on day-1. Same dose was continued till 1 month unless patient developed some adverse effect like persistent diarrhea or leukopenia, in which case dose was reduced accordingly. After 1st month, the dose was reduced to 1500 mg MMF or 1080 mg MMF Na until 3 months and then it was further reduced to 1000 mg MMF or 720 mg MMF Na/day and was maintained at same dose unless replaced or changed due to some adverse effect. If immunosuppression of patient on CSW was changed due to some reason, i.e. TAC to cyclosporine or MMF to azathioprine or everolimus, CS was continued in that patient.
All patients received intravenous (IV) basiliximab (simulect, Novartis pharm) 20 mg on day 0 via infusion just before surgery and again on the 4th post-operative day. All patients received parenteral methylprednisolone 500 mg IV infusion perioperatively. Thereafter patients in early CSW group received oral prednisolone 40 mg on day 1, which was tapered by 10 mg/day and finally stopped by day 5. Patients in CS group received oral prednisolone 40 mg/day on day 1, tapered to 20 mg/day by day 10, which was further tapered gradually to 5-7.5 mg/day by the end of 3 months.
In patients with graft dysfunction and suspected AR, kidney biopsy was carried out. Patients with acute cellular rejection (ACR) on biopsy were treated initially with parenteral methylprednisolone 500 mg daily for 3-5 days depending on the response. Steroid resistant rejection was defined as failure to respond to pulse steroids within a week of treatment. In such patients kidney biopsy was repeated before deciding about further treatment. Steroid resistant rejections were treated with IV thymoglobulin (r-ATG-Genzyme) 1.5 mg/kg/on alternate days for 3-5 doses. Patients with antibody mediated rejection (AMR) were treated with plasmapheresis (PP) and IV immunoglobulin (IVIG) 100 mg/kg after every PP. Patients who had features of mixed ACR and AMR received thymoglobulin along with PP. At least four sessions of PP and IVIG or PP and/thymoglobulin were given to patients with AMR or/mixed rejection respectively. Patients in CSW group, who developed AR, were continued on steroids after treatment. Steroids were initiated if patients developed acute tubular necrosis (ATN) or found to have interstitial inflammation or interstial fibrosis tubular atrophy on kidney biopsy. In addition, patients in whom immunosuppression was changed from TAC to cyclosporine or MMF to azathioprine or everolimus were also initiated on steroids.
Efficacy and safety
The primary efficacy parameter was the incidence of AR at 6 months. Both clinical and biopsy proven acute rejection (BPAR) were included for efficacy. All patients with suspected rejection underwent graft biopsy, unless contraindicated. Rejection was classified according to the modified Banff criteria.
Secondary efficacy endpoints included severity of BPAR, CS resistant rejection, incidence of AMR, renal function measured by serum creatinine and graft survival.
Safety parameters were adverse events such as leukopenia, occurrence of NODAT, infections, number of antihypertensive drugs, use of statins and patient survival.
Statistical analysis
The data was reported as mean ± standard deviation or median and range. Continuous variables were analyzed using Student's t test or the Wilcoxon rank sum test if the data was normally distributed. Categorical variables were analyzed using a Chi-square test or Fischer's exact test whenever appropriate. Survival analysis was performed by Kaplan Meier method. P < 0.05 was considered to be significant.
Results
The baseline demographics of patients are described in Table 1. There were 73 patients in CSW group and 67 patients in CS group. Patients on CSW regimen were younger and had more females as compared to CS group. More patients in CS group had diabetes as basic disease (50%) as compared to patients on CSW regimen (26%, P < 0.01), The mean follow-up was longer in CSW group (19.2 ± 5.7 months) as compared to CS group (12.5 ± 5 months, P = 0.01). There was no difference in donor parameters in two groups including HLA match.

Acute rejection
AR at 6 months, including clinical and biopsy proven rejection were significantly higher in early CSW group: 15 (20.5%) patients in CSW group had AR as compared to 5 (7.5%) in CS group (P = 0.02) [Table 2a]. In CSW group 3/15 (20%) patients had clinical rejections, as in two patients, biopsy tissue could not be obtained and in the third patient, biopsy was not done due to deranged coagulation parameters. However all three patients had >30% rise in serum creatinine and renal function returned to baseline after pulse methylprednisolone. All patients in CS group had biopsy proven rejections. The BPAR did not differ significantly between two groups-5 (7.5%) in CS versus 12 (16.5%) in CSW group (P = 0.1), Rejection free survival was significantly high in CS group as compared to CSW group [log rank, P = 0.02, Figure 1].

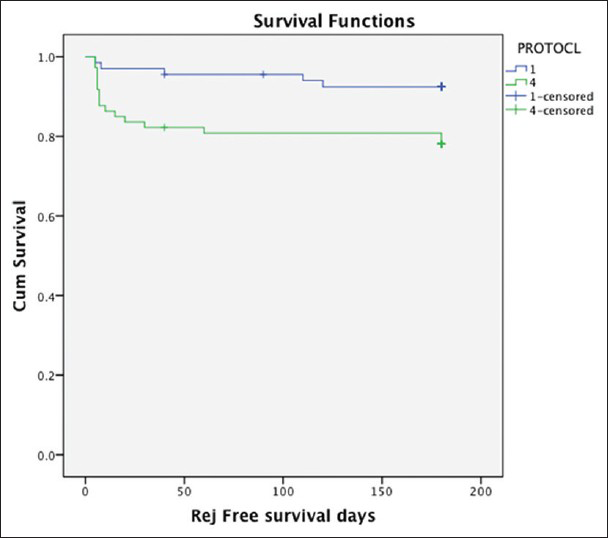
- Rejection free survival between corticosteroid withdrawal group (protocol 4) and corticosteroid (CS) group (protocol 1). Rejection free survival is significantly higher in CS group as compared to CSW (log rank,P = 0.02)
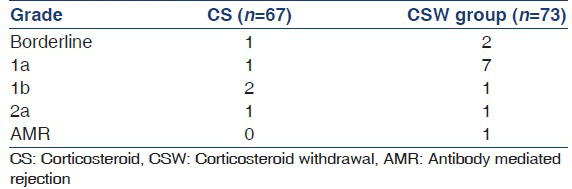
Most rejections were mild in both groups as shown in Table 2b. All patients had a complete response to pulse steroids in CS group and none of them developed CR till last follow-up. One patient in CS group without AR was found to have CR in biopsy. In patients with AR in CSW group; 4/15 patients were found to have CR in repeat biopsy. In addition, three other patients without AR were also found to have CR in their biopsies. AMR was seen in one patient in CSW group at 6 month, this patient responded to pulse steroids along with IVIG and PP, however he developed a chest infection and died.
There was no difference in TAC levels between the groups. The mean TAC levels were 12.3 and 12.7 on day 3, 13.2 and 12.4 on day 7 and 10.7 and 10 at 1 month in CS and CSW group respectively.
Most rejection episodes in CSW group occurred within a month (12/15), the average time to rejection was 24 days in CSW group as compared to 56 days in CS group.
Adverse events
NODAT was seen in three patients in CS group and two in CSW group. If we exclude patients with pre-existing diabetes from total patients then 3/33 (9%) patients in CS group developed NODAT as compared to 2/54 (3.7%) in CSW group, which was not significantly different (P = 0.09) [Table 3].

In CS group, 5 (7.5%) patients had infections, three of them had UTI. In one patient with recurrent pyelonephritis, graft nephrectomy was done at 6 months due to unresolved infection. One patient developed fungal pneumonia, which responded to treatment. The incidence of infections was significantly higher in CSW group, in which 15 (20.5%), patients had some infection (P = 0.02). Most of these patients had UTI, which responded to treatment, two patients had recurrent UTIs for which native kidney nephrectomy was carried out, one of these patient had ADPKD as basic disease and another had reflux nephropathy with VUR. One patient developed cytomegalovirus infection after 9 months and one patient developed BK Virus infection. Both these patients responded to treatment. Two patients developed serious chest infections, one improved after lobectomy for klebsiella pneumonia and other patient died due to chest infection after being treated for AMR.
Significantly higher number of patients switched from their original protocol in CSW regimen, 28 (38%) as compared to only 4 (6%) patients in CS regimen (P < 0.01) at the end of follow-up. In CS group, two patients were changed from MMF to azathioprine due to persistent loose motions; one was converted to everolimus and low dose TAC and fourth was changed from TAC to cyclosporine due to persistent tremors. In CSW group, majority of patients were converted to steroids after AR (15/28). Other patients were converted to steroids after ATN, increase proteinuria, inflammation or CR in biopsy, or if change from MMF to azathioprine or everolimus was carried out.
There was no difference in use of antihypertensive drugs and incidence of leukopenia between the groups, however statin use was significantly higher in CS group (64%) as compared to CSW group (45%, P = 0.02). No malignancy was reported in either group until last follow-up.
Renal function and survival
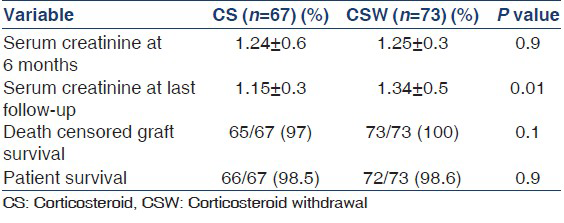
Serum creatinine was similar in two groups at 6 months (1.24 ± 0.6 mg/dl in CS and 1.25 ± 0.3 mg/dl in CSW, P = 0.9), however at the end of follow-up, mean serum creatinine was less in CS group as compared to CSW group (1.15 ± 0.3 mg/dl vs. 1.34 ± 0.5 mg/dl, P = 0.01). There was no difference in graft and patient survival at the end of follow-up between the groups [Table 4]. In CS group, two patients lost their graft, one due to renal vein thrombosis within 1st week, his graft nephrectomy was done and other patient developed recurrent pyelonephritis and graft nephrectomy was done at 6 months. No patient lost his graft till last follow-up in CSW group. One patient died in CS group due to cardiac event and one patient died in CSW group due to chest infection.
Discussion
In this study, we found that AR rates in our living donor kidney transplantation with early CSW regimen of basiliximab/TAC/MMF were significantly higher as compared to similar regimen with CS. However, the biopsy proven AR was not significantly different in two groups. The time to AR was earlier in steroid free group as compared to steroid based group (24 vs. 56 days). Majority of rejections in CSW group occurred within a month (12/15, 80%), as compared to only 2/5 (40%) rejection in CS group within a month. The mean TAC levels were similar in two groups in 1st month.
Initial studies, which have used cyclosporine alone or in combination with azathioprine, in which steroids were tapered and gradually stopped after 3 months, were associated with unacceptably high rates of rejections and graft loss.[567] Hence the initial enthusiasm of steroid withdrawal kidney transplant waned and this was not attempted for many years. The landmark Canadian multicenter study by Sinclair et al. randomized patients at 3 months to continue either on dual drug regimen of cyclosporine and steroids or stopped steroids. For initial 500-600 days, there was no significant difference between the groups; but after that there was an increased risk of graft loss in cyclosporine monotherapy group.[7]
Subsequently with introduction of MMF, it was shown that AR rates were lower than with azathioprine, when used in combination with steroids,[14] however a meta-analysis of early studies by Pascual et al. revealed higher rates of AR with CSW protocol even with the use of MMF along with TAC or cyclosporine, but graft loss was not significantly different[15] Our study also has similar findings, where AR is higher, but there is no difference in graft survival at least in the short term follow-up.
Subsequent to these early reports of higher AR rates with late steroid withdrawal and availability of better immunosuppressive agents, new attempts of steroid withdrawal with either rapid discontinuation of steroids (<7 days) or complete avoidance of steroids were made. Almost all these studies used induction with either IL-2 RA or ATG.[16171819]
Vincenti et al., in his randomized controlled trial compared three different regimens of basiliximab, cyclosporine and enteric coated MMF NA: No prednisolone, prednisolone till 7 days post-transplant versus maintenance prednisolone. There was a significant increase in biopsy proven AR in two prednisolone free group compared with maintenance prednisolone (P = 0.04) at 12 months. However, graft survival was similar in all three groups at 12 months.[17]
In another trial, by Vitko et al., where he compared TAC-MMF-prednisolone with TAC-MMF and basiliximab-TAC. There was a significant increase in rates of AR in steroid free groups (TAC-MMF 30.5%, TAC-Basiliximab 26.1%) as compared to TAC-MMF-steroid group (8.2%, P ≤ 0.001), but there was no difference in graft or patient survival. This study suggests that induction plus double therapy is probably required for success of steroid free regimen.[18]
In a recent report of Astella's prospective, multicenter, double blind, randomized controlled trial comparing early CSW to steroid continuation with a regimen of TAC, MMF and either IL-2 RA or ATG induction revealed higher rates of AR in CSW group as compared to steroid continuation group (12.8% vs. 22%, P = 0.02).[19] This recent report also included the borderline rejection, which was not included in previous results of the same trial, although AR (P = 0.04) as well as incidence of chronic allograft nephropathy was also high in the previous report also.[20]
However in other studies, there was no significant difference in AR when CNI was used in combination with MMF and antibody induction with or without steroids.[121321]
In a randomized controlled trial by Rostaing et al., in which he randomized 538 patients in two groups with regimen of DAC/TAC/MMF or TAC/MMF/steroid. At 6 months AR rates were 16.5% in both groups.[12] However in this study authors did not use IL-2 RA in the steroid group and mean TAC doses were higher in TAC/MMF/Steroid group as compared to DAC/MMF/TAC group, which might be the reason of similar rejections in both groups.
There is some evidence that using more potent induction agent like thymoglobulin might reduce the risk of AR.[202223] In subgroup analysis of study by Woodle et al., in CSW group the AR were lower in patients who received thymoglobulin as compared to those on IL-2RA.[20] Suresh Kumar et al., has shown in OPTN/UNOS data base that use of ATG as an induction agent was associated with better graft survival and reduced AR rates with a regimen of CNI/MMF/steroid free as compared with induction with Alemtuzumab or IL-2RA.[23] In our study, we have used basiliximab induction, which may be one of the reasons of higher rates of AR.
In our study, most of the rejection episodes in CSW group occurred within a month and these were mild rejections which responded to pulse steroids. Similar results have been seen in other studies also.[172021] It has been shown that early rejections have a better prognosis as compared to late rejections.[24]
In our study, there were some differences in baseline characteristics in two groups as patients in CSW group were younger and there were more females. This may be because younger recipients and females probably have chosen the CSW regimen more frequently, as it was not a randomized trial and patients decided about steroids themselves after counseling. Similarly number of diabetics was less in CSW group, which can be explained by younger age of recipients in CSW group.
The infection rate in this study was surprisingly higher in CSW group as compared to CS group. This may be explained by higher number of rejections in CSW group leading to more use of steroid pulses and higher immunosuppression. The incidence of NODAT was similar in both groups, which might be due to use of low doses of steroids in our CS group, as most of our patients were on 5 mg/day steroid at the end of 3 months. Similar incidence of diabetes has been seen in other studies also.[1820] Use of statins was more frequent in CSW group as compared to CS group.
In our study four patients with AR and another three patients without previous AR were found to have CR on biopsy in CSW group, which was higher than patients on steroids, where only one patient was found to have CR. This difference might get significant in long-term. Similar results have been reported in other studies also.[20] About one third of our patients returned back to steroid at the end of follow-up (mean 19 months) in CSW group, due to various reasons including AR (20%), ATN, inflammation in biopsy and CR. In USRDS data of 2007, about one fourth patients on steroid free regimen returned back on steroid at 1 year follow-up.[25]
In this short-term study, there was no difference in graft and patient survival, as has been reported in other studies also.[12161718192021] At last follow-up, the mean serum creatinine was higher in CSW group as compared to CS group (P < 0.01). A recent meta-analysis of steroid free kidney transplant found that this regimen is not associated with increased risk of graft failure or death, but higher risk of AR and rise in serum creatinine value.[26]
There are certain limitations to this study. First of all, this was a retrospective analysis of data at our center, which has led to some difference in baseline demography of recipients in two groups; however this was a single center study, so there was no difference in immunosuppressive protocols, all the patients were followed regularly and their data was collected at every visit. Another limitation was short-term follow-up, so even with higher creatinine at last follow-up; there was no difference in graft survival, which might become significant on longer follow-up. To address this issue a larger, prospectively designed study with long-term follow-up is required.
To summarize, this study of first kidney transplant recipient with living donors demonstrates that early CSW regimen with TAC/MMF and basiliximab induction is associated with higher rates of AR at 6 months as compared to patients on similar regimen with steroids. However, most of these rejections were mild, occured early and responded to pulse steroids. In this study patients were more HLA mismatched as we included both spouse and one haplotype matched donors and patients received basiliximab induction. Use of thymoglobulin for induction and selecting one haplotype matched donors or HLA identical donors for such regimen might reduce AR rates. There was no significant difference in incidence of NODAT. At 6 months, renal function as measured by s. creatinine was similar in both groups. Graft and patient survival was also similar in groups at the end of follow up.
To conclude early CSW regimen in living donor first kidney transplant recipients was associated with higher overall AR at 6 months, but similar graft and patient survival.
Acknowledgments
The authors would like to thank Dr. Padam Singh, Dr. Beena Bansal for statistical support.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Overview of the side effects of corticosteroid therapy. Clin Exp Rheumatol. 1991;9(Suppl 6):19-20.
- [Google Scholar]
- Risk factors for and management of post-transplantation cardiovascular disease. BioDrugs. 2001;15:261-78.
- [Google Scholar]
- Low dose long-term corticosteroid therapy in rheumatoid arthritis: An analysis of serious adverse events. Am J Med. 1994;96:115-23.
- [Google Scholar]
- Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55:420-6.
- [Google Scholar]
- Steroid-free immunosuppression in cyclosporine-treated renal transplant recipients: A meta-analysis. J Am Soc Nephrol. 1993;4:1300-5.
- [Google Scholar]
- A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol. 2000;11:1910-7.
- [Google Scholar]
- Low-dose steroid therapy in cyclosporine-treated renal transplant recipients with well-functioning grafts. CMAJ. 1992;147:645-57.
- [Google Scholar]
- A multicenter pilot study of early (4-day) steroid cessation in renal transplant recipients under simulect, tacrolimus and sirolimus. Am J Transplant. 2005;5:157-66.
- [Google Scholar]
- Early steroid withdrawal therapy in renal transplant recipients: A steroid-free sirolimus and CellCept-based calcineurin inhibitor-minimization protocol. Clin Transplant. 2007;21:101-9.
- [Google Scholar]
- Rapid discontinuation of steroids in living donor kidney transplantation: A pilot study. Am J Transplant. 2001;1:278-83.
- [Google Scholar]
- Three-year observational follow-up of a multicenter, randomized trial on tacrolimus-based therapy with withdrawal of steroids or mycophenolate mofetil after renal transplant. Transplantation. 2006;82:55-61.
- [Google Scholar]
- Corticosteroid-free immunosuppression with tacrolimus, mycophenolate mofetil, and daclizumab induction in renal transplantation. Transplantation. 2005;79:807-14.
- [Google Scholar]
- Steroid-withdrawal at 3 days after renal transplantation with anti-IL-2 receptor alpha therapy: A prospective, randomized, multicenter study. Am J Transplant. 2004;4:803-10.
- [Google Scholar]
- A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation. 1996;61:1029.
- [Google Scholar]
- Spanish Group for Evidence-Based Medicine in Renal Transplantation. Steroid withdrawal in renal transplant patients on triple therapy with a calcineurin inhibitor and mycophenolate mofetil: A meta-analysis of randomized, controlled trials. Transplantation. 2004;78:1548-56.
- [Google Scholar]
- Steroid-free immunosuppression after kidney transplantation with antithymocyte globulin induction and cyclosporine and mycophenolate mofetil maintenance therapy. Transplantation. 2002;73:1527.
- [Google Scholar]
- A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8:307-16.
- [Google Scholar]
- Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: Results of the Atlas study. Transplantation. 2005;80:1734-41.
- [Google Scholar]
- Acute rejection characteristics from a prospective, randomized, double-blind, placebo-controlled multicenter trial of early corticosteroid withdrawal. Transplantation. 2013;95:573-9.
- [Google Scholar]
- A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248:564-77.
- [Google Scholar]
- Multicenter randomized prospective trial of steroid withdrawal in renal transplant recipients receiving basiliximab, cyclosporine microemulsion and mycophenolate mofetil. Am J Transplant. 2003;3:306-11.
- [Google Scholar]
- Long-term immunosuppression, without maintenance prednisone, after kidney transplantation. Ann Surg. 2004;240:510-6.
- [Google Scholar]
- Influence of induction modality on the outcome of deceased donor kidney transplant recipients discharged on steroid-free maintenance immunosuppression. Transplantation. 2012;93:799-805.
- [Google Scholar]
- Early versus late acute rejection episodes in renal transplantation. Transplantation. 2003;75:204-8.
- [Google Scholar]
- The success of continued steroid avoidance after kidney transplantation in the US. Am J Transplant. 2009;9:2768.
- [Google Scholar]
- Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010;89:1-14.
- [Google Scholar]







