Translate this page into:
Eculizumab Dosing in Infants
Address for correspondence: Dr. G. Filler, Department of Pediatrics, Children's Hospital - London Health Sciences Centre, University of Western Ontario, 800 Commissioner's Road East, Rm B1-138A, London, ON N6A 5W9, Canada. E-mail: guido.filler@lhsc.on.ca
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Eculizumab is the therapy of choice for patients with atypical hemolytic uremic syndrome (aHUS). Dosing recommendations stem from two trials: one retrospective trial (19 children and 5 infants) and one prospective trial (22 patients and 5 infants). This case report highlights the need for more precise dosing recommendations in children, particularly in infants, and for smaller vials of the medication to facilitate more precise dosing. Such changes would ensure that adverse events are minimized and that the children with aHUS who are treated with eculizumab experience an optimal clinical response.
Keywords
Atypical hemolytic uremic syndrome
biologic
eculizumab
dosing
pediatrics
Introduction
Atypical hemolytic uremic syndrome (aHUS) is an immune disorder stemming from dysregulation of the alternative complement pathway, leading to complement overactivation. The disease presents as a combination of thrombotic microangiopathy (TMA), thrombocytopenia, and renal failure. Although rare with an estimated prevalence of approximately 0.11 new cases/million population/year,[12] it has a very high mortality and morbidity that extends far beyond the initial presentation period.[3] The approval of eculizumab (Soliris, Alexion Pharmaceuticals, Inc., Cheshire, CT, USA) on September 23, 2011, as an orphan drug marked a breakthrough for this condition, as it was the first Food and Drug Administration-approved treatment for aHUS; an untreatable disease suddenly became treatable. International guidelines place eculizumab as a first-line treatment in patients with a clinical diagnosis of aHUS (confirmation of a complement mutation is not required) that should be initiated within 24–48 h of onset or admission.[4] This quick initiation is to achieve the best outcome with regard to ultimate renal recovery and to diminish the risk of early progression to end-stage renal disease.[567] However, its dosing presents a problem in pediatrics. The current dosing recommendations on the eculizumab product label[89] are weight based in increments of 5 kg and then 10 kg: 5–9.9 kg, 10–19.9 kg, 20–29.9 kg, 30–39.9 kg, and 40 kg and over. The approval for the aHUS indication in children was originally made based on one retrospective chart review study outside of a controlled clinical trial setting in 19 children under the age of 18 years, with 15 of those under the age of 12 years of age and 5 under 2 years of age.[1011]
One prospective pediatric study has been published since eculizumab was approved by the major regulatory agencies to treat aHUS; the intent-to-treat population of this study was 22, while only 19 completed 26 weeks of treatment. Of these children, only one was younger than 1 year of age (personal communication), five were below 2 years of age, and 18 were below 12 years of age.[12] The composition of both of these study cohorts suggests that the dosing for children, particularly for newborns, infants, and toddlers, was extrapolated from a handful of patients. Since this period brings rapid changes in growth and ontogeny in drug disposition, pediatric dosing recommendations must be exact, and medications for children are typically dosed either per kilogram of body weight or per m2 of the child's body surface area (BSA) rather than in vague weight increments. We detail the case of the youngest patient in Canada with aHUS to be treated with eculizumab and describe the associated dosing challenges.
Case Report
An 8-month-old previously healthy, fully immunized, formula-fed female presented to the emergency room with a short history of an upper respiratory tract infection (confirmed respiratory syncytial virus), feeding intolerance, emesis (nonbilious and nonbloody), reduced urine output, no diarrhea, and no contact with farm animals or cow milk products. One day after her admission, while afebrile, she was somnolent, pale, had slight jaundice, and mild dehydration. Her urine output did not improve following rehydration. She was transferred to a tertiary care center, where testing revealed the blood parameter levels as shown in Table 1.
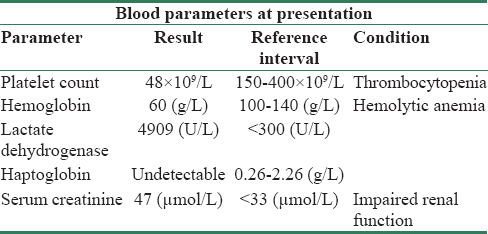
Based on these results, she was diagnosed with systemic TMA.
Her urinary sediment was very active. Her ADAMTS 13 (a disintegrin and metalloproteinase with a thrombospondin Type 1 motif, member 13) activity was >69% (reference interval 41%–130%), ruling out thrombocytopenic purpura, and her stool was negative for Escherichia coli O157: H7, ruling out Shiga toxin-producing E. coli-HUS. The differential diagnosis was therefore aHUS. Her complement C3 activity was normal, but her CH50 activity was undetectable. The genetic workup confirmed the presence of a pathogenic variant (c.3546G>C [Arg1182Ser]) and of a variant of uncertain significance (C.3148A>T [Asn1050Tyr]) in the complement factor H (CFH) gene (laboratory of Dr. Christoph Licht, Hospital for Sick Children, Toronto, Canada), while there were no anti-CFH antibodies. Treatment consisted of plasma infusion therapy (up to 55 ml/FFP/kg) and four treatments with plasma exchange with up to 1.5 times of the patient's plasma volume. She also received two blood transfusions. Platelets, urine output, and creatinine normalized. No steroids or other agents were given. Three weeks later, she relapsed in association with an upper respiratory tract infection. As there was no antibody titer detectable at initial presentation, this was not reevaluated. At this time, her hemoglobin dropped to 67 g/L, her platelets dropped to 52 × 109/L, lactate dehydrogenase rose to 1266 U/L, haptoglobin was undetectable, and she required three transfusions. After receiving a meningococcal conjugate vaccine, she was started on eculizumab, in accordance with the consensus guidelines. With a weight of 9.17 kg, she was initially given one 300 mg dose for the 1st week of induction, then 300 mg 1 week later and 300 mg every 3 weeks during the maintenance phase, as per the manufacturer's instructions. Her platelet count normalized after the first two treatments and her haptoglobin normalized after 14 weeks. Interestingly, the patient would experience feeding intolerance and vomiting on day 16 following each treatment. Treatment was accelerated to 18-day and then 17-day intervals without any improvement in her day 16 symptoms; the dosing interval was therefore shortened to 14 days. Finally, the patient's vomiting subsided with 14-day treatment intervals, and for the first time, after not having gained any weight from the time of presentation, she gained some weight to 9.4 kg following the first 14-day treatment interval. Her weight dropped from the 92nd to the 52nd percentile. Her CH50 complement activity remained undetectable. The results of tests to measure eculizumab concentration are currently pending. Her cystatin C estimated glomerular filtration rate remains abnormal at 71 ml/min 1.73 m2 following 8 months of therapy [Figure 1].
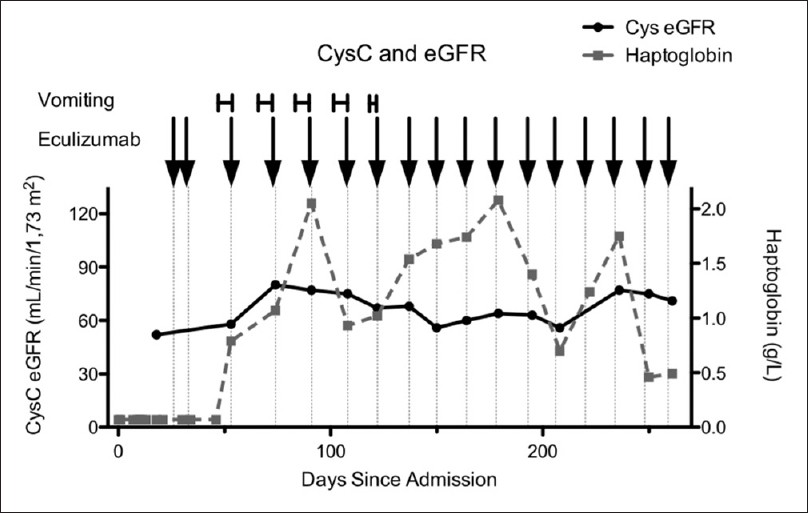
- Chronological cystatin C estimated glomerular filtration rate and haptoglobin measurements from the time of admission. Vomiting is marked according to its duration and eculizumab treatments are marked with arrows
Discussion
Great strides have been made within the last 15 years in understanding the pathophysiology of aHUS, and scientists are still elucidating the genetic background of this disease by continuing to identify new genetic mutations associated with aHUS. The publication of comprehensive international guidelines for diagnosing and treating pediatric aHUS in January of 2016 in Pediatric Nephrology[4] also represents a significant advancement in physicians’ abilities to properly diagnose aHUS in children. Since, until very recently, an aHUS diagnosis entailed a grim outlook and often death, new guidelines and a new treatment will most likely result in a greater number of diagnoses that will be made more quickly and will be more accurate. In the prospective pediatric eculizumab trial by Greenbaum et al.,[12] a post hoc analysis examining the children who did and did not meet criteria for the primary endpoint at 26 weeks, which was defined as a complete TMA response, revealed a stark difference in the median age at first infusion. The median age at first infusion of children who achieved the primary endpoint was 7.5 years, while the median age of those who did not was 2.0 years. This suggests that the younger children tended to be underdosed and that eculizumab has a shorter half-life in children, so they were less likely to achieve the primary endpoint when dosed according to the current guidelines. This case study, where eculizumab dosing in the <10 kg weight category was empirically escalated, eventually resulting in a clinical remission, highlights the need for more infant data to refine the current pediatric eculizumab dosing guidelines. With the likelihood that the identified prevalence of aHUS will increase in the coming years, it is important to refine eculizumab dosing in children, particularly in younger children. In addition to recruiting more patients and collecting more data to establish more precise dosing per kilogram of body weight or per m2 of BSA, smaller vials should be produced to implement more accurate dosing. Vials of 50 mg, 100 mg, or even 150 mg would be highly beneficial to clinicians. In the absence of additional pediatric pharmacokinetic studies and precise guidelines, physicians should observe the patient's clinical response and adjust the patient's dose to minimize any adverse effects. The clinician should also employ pharmacokinetic monitoring if the patient's clinical response is insufficient and adjust the dose accordingly.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
References
- An audit analysis of a guideline for the investigation and initial therapy of diarrhea negative (atypical) hemolytic uremic syndrome. Pediatr Nephrol. 2014;29:1967-78.
- [Google Scholar]
- Genetics and outcome of atypical hemolytic uremic syndrome: A nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554-62.
- [Google Scholar]
- The long-term outcomes of atypical haemolytic uraemic syndrome: A national surveillance study. Arch Dis Child. 2016;101:387-91.
- [Google Scholar]
- An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15-39.
- [Google Scholar]
- Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169-81.
- [Google Scholar]
- Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061-73.
- [Google Scholar]
- Insights from the use in clinical practice of eculizumab in adult patients with atypical hemolytic uremic syndrome affecting the native kidneys: An analysis of 19 cases. Am J Kidney Dis. 2014;63:40-8.
- [Google Scholar]
- Soliris (Eculizumab): EU Summary of Product Characteristics. Available from: http://www.ema.europa.eu/docs/en-GB/document-library/EPAR-product-information/human/000791/WC500054208.pdf
- [Google Scholar]
- 2011. Available from: http://www.accessdata.fda.gov/drugsatfda-docs/label/2011/125166s172lbl.pdf
- Eculizumab therapy for atypical hemolytic uremic syndrome in pediatric patients: Efficacy and safety outcomes from a retrospective study. Haematologica. 2011;96(Suppl 2):165.
- [Google Scholar]
- Eculizumab therapy for pediatric patients with atypical hemolytic uremic syndrome: Efficacy and safety outcomes of a retrospective study. Haematologica. 2012;97(Suppl 2):479.
- [Google Scholar]
- Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701-11.
- [Google Scholar]







