Translate this page into:
Effect of aqueous extract of Tribulus terrestris on oxalate-induced oxidative stress in rats
Address for correspondence: Dr. S. K. Singla, Department of Biochemistry, Panjab University, Chandigarh - 160 014, India. E-mail: pubiochem14@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The present study was aimed at studying the effect of Tribulus terrestris on different parameters of oxidative stress and gene expression profiles of antioxidant enzymes in renal tissues of male wistar rats after induction of hyperoxaluria. The animals were divided into three groups. The animals in group I (control) were administered vehicle only. In group II, the animals were treated with ethylene glycol (hyperoxaluric agent) and those in group III were administered T. terrestris plant extract in addition to ethylene glycol. All treatments were continued for a period of seven weeks. Ethylene glycol feeding resulted in hyperoxaluria as well as increased excretion of calcium and phosphate. Serum creatinine, uric acid and blood urea nitrogen levels were also altered in hyperoxaluric animals. Various oxidative stress parameters viz. lipid peroxidation and activity of antioxidant enzymes were used to confirm the peroxidant state. Reverse transcription-polymerase chain reaction (RT-PCR) analysis was used to confirm whether steady-state transcription level of different antioxidant enzymes was altered. T. terrestris significantly reduced the excretion of oxalate, calcium, and phosphate along with decreased levels of blood urea nitrogen, uric acid and creatinine in serum. T. terrestris also reduced hyperoxaluria- caused oxidative stress, and restored antioxidant enzyme activity and their expression profile in kidney tissue. Histological analysis depicted that T. terrestris treatment decreased renal epithelial damage, inflammation, and restored normal glomerular morphology.
Keywords
Blood urea nitrogen
creatinine
hyperoxaluria
Tribulus terrestris
urea
Introduction
Stone formation in the kidney is one of the oldest and the most widespread diseases known to man. It is a common chronic disorder affecting 10-15% population worldwide. Chemically, stones contain calcium, magnesium ammonium phosphate, uric acid, and cysteine. Among these, calcium-containing stones constitute about 75% of all urinary calculi and can exist as either calcium oxalate or calcium phosphate (Apatite), as well as a mixture of both.[1] Hyperoxaluria is one of the causes of oxalate urinary stones. Urinary oxalate is directly related to diet, but the majority of oxalate is produced via metabolism of glyoxylic acid.[2] This occurs due to deficiency of liver-specific peroxisomal enzyme alanine/glyoxylate aminotransferase.[3]
Studies have shown that renal cellular exposure to oxalate and/or calcium oxalate crystals leads to formation of reactive oxygen species and also development of oxidative stress followed by injury and inflammation.[4] Moreover, it appears to play a significant role in stone formation. Stones in the urinary tract can be detected by X-ray, intravenous pyelography, ultrasound, and computed tomography (CT) scan. In addition to open surgery, non-invasive techniques, extracorporeal shock wave lithotripsy (ESWL), and percutaneous nephrolithotomy are used for the management of stones in the upper urinary tract. All these techniques cause undesirable effects such as hemorrhage, hypertension, tubular necrosis, and subsequent fibrosis of the kidney leading to cell injury and recurrence of renal stone formation.[5] Since these techniques only remove the stones but not their cause, there is a need for alternative management of urolithiasis. Animal model studies show that treatments with antioxidants and free radical scavengers reduce oxalate or calcium oxalate-induced injury.[4] Tribulus terrestris is a natural herb, also known as Puncture vine or Gokhru, used for treating several diseases and found in many tropical and moderate areas of the world, including the US and Mexico, Mediterranean region, and throughout Asia. T. terrestris is aphrodisiac, antihypertensive, diuretic, and antiurolithiatic in nature.[6–8] In India, many Ayurvedic practitioners use aqueous extract of this plant in the treatment of kidney stones. From previous studies, it was found that aqueous extract of T. terrestris produced inhibitory effect toward calcium oxalate crystallization.[9] Therefore, the present study was undertaken to observe the effect of aqueous extract of T. terrestris on oxidative stress in the kidney of rats, which was induced by ethylene glycol with an aim to establish the mode of its action.
Materials and Methods
Tribulus terrestris was purchased from the local market and authenticated with the specimens maintained at University Institute of Pharmaceutical Sciences (UIPS), Panjab University, Chandigarh. Fruits of the plant were used in the present study. All reagents used were of analytical grade of Merck (Germany) and SRL Chemicals (India). A reverse transcription-polymerase chain reaction (RT-PCR) kit was purchased from Invitrogen, USA.
The fruit of the plant was weighed and soaked in double distilled water (5% w/v) overnight at 4°C. The extract was filtered through muslin cloth and subjected to centrifugation at 3,000 rpm for 10 minutes at 4°C in cold centrifuge .The supernatant thus obtained was referred to as aqueous extract. Male Wistar rats were divided into groups I, II, and III, containing 10 animals each. Ethylene glycol was used to induce hyperoxaluria in rats[10] Group I served as control and received regular rat feed and drinking water ad libitum. Group II was administered hyperoxaluric agent – 0.6% ethylene glycol (EG) for 7 weeks in drinking water. Group III was given 5% aqueous extract of T. terrestris in addition to ethylene glycol. The animals were acclimatized to standard laboratory conditions and the animal care and experimental protocols were in accordance with Institutional Animal Ethics Committee (IAEC), Panjab University, Chandigarh.
All animals were kept in individual metabolic cages and 24-h urine samples were collected one day before sacrificing the animals. Urinary calcium,[11] phosphate,[12] and oxalate[13] levels were estimated. Blood was collected from orbital sinus under anesthetic conditions and animals were sacrificed by cervical decapitation. Serum was separated by centrifugation at 3,000× g for 15 minutes and analyzed for creatinine,[14] urea nitrogen,[15] and uric acid.[16]
A 10% kidney homogenate was prepared in 0.1M Tris buffer (pH 7.4) and it was used for assaying lipid peroxidation[17] and antioxidant enzymes viz glutathione S-transferase (GST)[18] and superoxide dismutase (SOD).[19]
For gene expression studies, total RNA was isolated from rat kidney using Trizol reagent (Gibco BRL, UK). Single-stranded complementary DNA (cDNA) was synthesized from 1 μg mRNA using oligo(dT)12-18 as primers and Avian Myeloblastosis Virus (AMV) reverse transcriptase. The amplification of different antioxidant enzymes was carried out by using specific primers, viz GST (forward primer 5’-CTG CAG CTG GAG TAG AGT TT-3’; reverse primer 5’-TTG TAT TTG GTG GCA ATG TA-3’, 169 bp) and SOD (forward primer 5’-GCA GAA GGC AAG CGG TGA AC-3’; reverse primer 5’-TAG CAG GAC AGC AGA TGA GT-3’, 446 bp).[20–24] Glyceraldehyde-3- phosphate dehydrogenase (GAPDH) was chosen as house-keeping gene (forward primer 5’-GAA CGG GAA GCT CAC TGG CAT-3’; reverse primer 5’-GTC CAC CAC CCT GTT GCT GTA-3’, 197 bp). Fifty microliter of PCR reaction mixture consisted of 25 μl of 2X reaction mix provided with the superscript III first-strand synthesis system (Invitrogen), 1 μg cDNA, 1 μl each of 10-μM forward and reverse primers, 1 μl of Taq mix enzyme, and RNAse-free water. The RT-PCR conditions were as follows: (1) reverse transcription, 30 min, 55°C; (2) initial activation step, 2 minutes at 94°C; (3) three-step cycling (34 cycles for GAPDH, 30 cycles for SOD and GST), each cycle consisting of denaturation for 45 sec at 94°C followed by annealing for 45 sec at 64°C for GAPDH, 55.5°C for SOD, 50°C for GST, and extension for 1 min at 68°C. The resulting PCR products were resolved on 1% agarose gels. The bands were identified based on the product size using 100-bp ladder. The PCR products were quantitated with densitometry scanning using Scion Image Software (Scion Incorporation, USA). The values were expressed as percentages with respect to control.
For histopathological studies, the kidneys were removed and sections were fixed in 10% buffered formalin solution (pH 7). The tissues were dehydrated and embedded with paraffin wax (68°C). The paraffin sections were then cut and finally stained with Delafield's Hematoxylin and Eosin (H and E) staining.
All data were expressed as mean±SD (n=10). Statistical analysis was performed by using one-way analysis of variance (ANOVA) followed by post hoc test for ascertaining significant difference. Statistical significance of the results was calculated at P<0.05.
Results
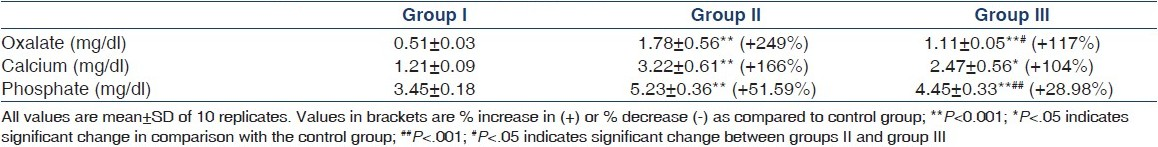
To assess the induction of hyperoxaluria, urinary excretion of oxalate was determined. Following the administration of ethylene glycol, excretion of oxalate, calcium and phosphate in urine increased significantly in group II as compared to control [Table 1]. However, T. terrestris treatment (group III) significantly decreased the urinary oxalate, calcium, and phosphate excretion as compared to that in group II.
A significant increase in creatinine, blood urea nitrogen, and uric acid levels in the serum was also observed in group II animals as compared to control [Table 2]. However, administration of T. terrestris significantly lowered the levels of creatinine, blood urea nitrogen, and uric acid in group III animals. It is evident from Table 3 that lipid peroxidation was increased significantly in hyperoxaluric animals (group II). Lipid peroxidation as measured by thiobarbituric acid reactive substances (TBARS) such as malondialdehyde was found to increase by 59.42%. However, a significant decrease was observed in the malondialdehyde value after treatment with aqueous extract of T. terrestris, as compared to hyperoxaluria-induced animals. Figure 1 shows the gene expression of SOD and GST measured by RT-PCR using gene-specific primers. Ethylene glycol exposure decreased the gene expression of both SOD and GST as assessed by RT-PCR analysis [Figures 2a and 3a]. However, T. terrestris administration reversed the changes in gene expression of both SOD and GST. As far as results of enzyme activity are concerned, the activity of both SOD and GST enzymes was significantly parallel to gene expression pattern [Figures 2b and 3b].



- Agarose gel electrophoresis of RT-PCR products from total RNA isolated from different groups using Trizol. L- ladder, C- control, GP II- group II, GP III- group III

- (a) Densitometric scanning of SOD following RT-PCR programme using Scion Image Software in different groups. Values in bracket are % increase (+) or decrease (-) with control group. Data are mean±SD of ratios of intensity of each gene divided by that of GAPDH gene (n=10). (b) Activity of SOD in different groups of animals. Values in bracket are % increase (+) or decrease (-) compared to the control group. **P<0.001, *P=0.001 indicate significant change in comparison with control group. ##P<0.001, #P<0.05 indicate significant change between groups II and III

- (a) Densitometric scanning of GST following RT-PCR programme using Scion Image Software in different groups. Values in bracket are % increase (+) or decrease (-) with control group. Data are mean±SD of ratios of intensity of each gene divided by that of GAPDH gene (n=10). (b) Activity of GST in different group of animals. Values in bracket are % increase (+) or decrease (-) compared to control group. **P<0.001, *P=0.001 indicate significant change in comparison with control group. ##P<0.001, #P<0.05 indicate significant change between groups II and III
Renal histology of urolithiatic animals [Figure 4b (i and ii)] also showed distorted morphology in contrast to that in the control group [Figure 4a]. The renal tubules lost their intact structure and shrunken lumen was observed. In addition to this morphological alteration, the histology of animals in this group has marked inflammation and hemolysis, as shown by arrows. The cells were distorted and signs of edema could be observed at several instances in their histology. However, the animals in group III showed restored morphology as compared to those in group II [Figure 4c]. The signs of inflammation were reduced and no hemolysis and edema were observed. The animals in this group showed normal size of glomerulus although loss of intact renal tubules was still prevalent.

- Renal histological analysis stained with hematoxylin and eosin (40×). ‘a’ is the kidney of control rat showing normal structure; ‘b’ is the kidney section of urolithiatic rats, with arrows showing areas of inflammation and hemolysis; ‘c’ is the kidney of group III (EG+ Tribulus terrestris) rats showing near normal renal structure
Discussion
Hyperoxaluria, defined as excessive urinary oxalate, is a common abnormality found in patients with calcium oxalate stones. In the present study, ethylene glycol (EG) was used for producing hyperoxaluria in male Wistar rats. The principal target organ following oral exposure to EG is the kidney;[25] moreover, evidences from previous studies indicated that administration of EG caused renal stone formation by increasing hyperoxaluria.[10] Increased urinary calcium is a factor favoring the nucleation and precipitation of calcium oxalate or calcium phosphate from urine,[26] and subsequent crystal growth results in stone formation. Increased urinary phosphate excretion along with oxalate stress seems to provide an environment appropriate for stone formation by forming calcium phosphate crystals, which epitaxially induces calcium oxalate deposition.[27] But treatment with aqueous extract of T. terrestris restored the phosphate level, thus reducing the risk of stone formation. Urolithiasis also decreases the glomerular filtration rate due to obstruction in outflow of urine by stones in the urinary system. Due to this, waste products, particularly nitrogenous substances such as urea, creatinine, and uric acid are accumulated in the blood.[28] Uric acid is also known to promote calcium oxalate crystal growth because of a predominance of uric acid crystals in calcium oxalate stones.[29] However, treatment with T. terrestris caused diuresis,[30] hastened the process of dissolving the preformed stones, and increased the excretion of urea, creatinine, and uric acid as well as normalized the kidney functions.
We assessed the effect of hyperoxaluria on rat's kidney by estimating oxidative stress markers. According to previous studies, oxalate is found to promote oxidative stress by inducing production of free radical in renal tissue.[31] Lipid peroxidation is the process of oxidative degradation of polyunsaturated fatty acids and causes impaired membrane function and structural integrity. In the present studies, lipid peroxidation was found to be significantly increased during hyperoxaluria. T. terrestris reduced the levels of free radicals responsible for lipid peroxidation, and thus decreased the level of malondialdehyde, end product of lipid peroxidation. This shows that T. terrestris has the potential of scavenging free radicals and reducing oxalate-induced free-radical damage, which is also evident from histological analysis. Our results revealed that hyperoxaluria also reduced the SOD and GST activities and their expression at the mRNA level in kidney tissues. The rebalancing of elevated antioxidant enzyme's activity and their gene expression by T. terrestris treatment further substantiated the protective nature of this plant extract against free radical-induced oxidative stress.
Oxalate-generated free radicals disrupt the structural integrity of the membranes in renal epithelial cells.[32] It has also been observed that human kidney with acute or chronic oxalosis shows inflammation and fibrosis.[33] Inflammation in kidney tissue has been found to recruit macrophages to sites of crystal deposition, which reduced renal crystal burden and acted as an early defense against crystal deposition.[34] In our studies, the damage to the glomerulus and its capsule, inflammation, and hemolysis following oxalate exposure was also more prevalent in hyperoxaluric animals, which might have been caused by high oxalate itself or its derivatives but T. terrestris-treated hyperoxaluric animals restored the normal morphology of glomeruli. This can be explained by antioxidant properties of T. terrestris, which reduce free radical damage caused by oxalate.
Therefore, it is clear from the studies that T. terrestris has the potential to normalize the peroxidant status and gene expression of antioxidant enzymes viz SOD and GST. In conclusion, T. terrestris, being an extraneous antioxidant, reduced oxidative stress, maintained proper renal functioning, and reduced renal injury.
The authors thanks the Chairperson, Department of Biochemistry, Panjab University, Chandigarh, India, for providing facilities to carry out this research.
Source of Support: Nil
Conflict of Interest: None declared.
References
- The relation of clinical catastrophes, endogenous oxalate production and urolithiasis. Clin Chem. 1990;36:1717-30.
- [Google Scholar]
- Peroxisomal alanine glyoxylate aminotransferase deficiency in primary hyperoxaluria type 1. Fed Eur Biochem Soc Lett. 1986;201:20-4.
- [Google Scholar]
- Hyperoxaluria induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33:349-57.
- [Google Scholar]
- A contemporary evaluation of the auditory hazard of extracorporeal shock wave lithotripsy. Urology. 2007;70:898-9.
- [Google Scholar]
- Aphrodisiac properties of Tribulus terrestris extract (protodioscin) in normal and castrated rats. Life Sciences. 2002;71:1385-96.
- [Google Scholar]
- Antihypertensive and vasodilator effect of methanolic and aqueous extract of Tribulus terrestris in rats. J Ethanopharmacol. 2002;104:3351-5.
- [Google Scholar]
- Evaluation of antilithiatic activity of Tribulus terrestris. Pharmaceutical Biol. 1994;32:217-24.
- [Google Scholar]
- Herbal extracts of Tribulus terrestris and Bergenia ligulata inhibit growth of calcium oxalate monohydrate crystals in vitro. J Crystal Growth. 2005;275:1403-8.
- [Google Scholar]
- Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol. 2005;289:536-46.
- [Google Scholar]
- A modification of colorimetric phosphorus determination for use with photoelectric colorimeter. J Lab Clin Med. 1941;27:955-60.
- [Google Scholar]
- An improved colorimetric procedure for urine oxalate. Clin Chim Acta. 1972;36:127-32.
- [Google Scholar]
- Interpretation and techniques Clin Chem. Philadelphia: Lea and Febiger; 1983.
- Clinical chemistry Principles and Technics. New York: Harper and Row; 1968. p. :268.
- New enzymatic method for serum uric acid at 500 nm. Clinical Chem. 1978;24:1908-1911.
- [Google Scholar]
- Glutathione-S-Transferase,The first enzymatic step in mercaptouric acid formation. J Biol Chem. 1974;249:7130-9.
- [Google Scholar]
- Generation of superoxide radical during autooxidation of hydroxylamine and assay for superoxide dismutase. Arch Biochem Biophy. 1978;186:189-215.
- [Google Scholar]
- Expression of extra cellular gluthaione peroxidase type 5 in male reproductive tract. Mol Human Reprod. 1998;4:841-8.
- [Google Scholar]
- Mammalian oviduct and protection against free oxygen radicals: Expression of genes encoding antioxidant enzymes in human and mouse. Eur J Obstet Gynecol Reprod Biol. 2000;89:1-4.
- [Google Scholar]
- Increased mRNA levels of Mn-SOD and Catalase in embryos of diabetic rats from a malformation resistant strain. Diabetes. 2000;49:101-7.
- [Google Scholar]
- Gene expression profiles of cultured rat cardiomyocytes (H9C2 cells) in response to arsenic trioxide at subcytotoxic levels and oxidative stress. J Health Sci. 2006;52:512-21.
- [Google Scholar]
- Genomic analysis of the rat lung following elemental mercury vapour exposure. Toxicol Sci. 2003;74:174-81.
- [Google Scholar]
- International programme on chemical safety (IPCS). Ethylene glycol: Human Health Aspects. In: Concise International Chemical Assessment Document 45. Geneva: World Health Organization; 2002. p. :4-5.
- [Google Scholar]
- The cause of idiopathic calcium disease: Hypercalciuria or hyperoxaluria? Nephron. 1980;26:105-10.
- [Google Scholar]
- An improved automated procedure for determination of calcium in biochemical specimen. Anal Biochem. 1967;18:521.
- [Google Scholar]
- Chemical tests in kidney disease. In: Text Book of Medical Laboratory Technology. Mumbai: Bhalani Publishing House; 1994. p. :118-32.
- [Google Scholar]
- Experimental evaluation of diuretic action of herbal drug (Tribulus terrestris) on albino rats. J Res Edu Ind Med. 1991;10:19-21.
- [Google Scholar]
- Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol Res. 2002;30:35-47.
- [Google Scholar]
- Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: Effect of antioxidants. Urol Res. 2003;31:3-9.
- [Google Scholar]
- Endocytosis of calcium oxalate crystals and proliferation of renal tubular epithelial cells in a patient with type 1 primary hyperoxaluria. J Urol. 1992;148:1517-9.
- [Google Scholar]







