Translate this page into:
Efficacy and Safety of Different Durations of Dual Antiplatelet Therapy for Acute Coronary Syndrome in Patients with Chronic Kidney Disease: A Systematic Review
Corresponding author: Alius Cahyadi, Department of Internal Medicine, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, North Jakarta, Indonesia. E-mail: alius.cahyadi@atmajaya.ac.id
-
Received: ,
Accepted: ,
How to cite this article: Suryohusodo AA, Cahyadi A, Tjhin C, Iryaningrum MR. Efficacy and Safety of Different Durations of Dual Antiplatelet Therapy for Acute Coronary Syndrome in Patients with Chronic Kidney Disease: A Systematic Review. Indian J Nephrol. doi: 10.25259/IJN_523_2024
Abstract
Background
In this systematic review, we evaluated the efficacy and safety of different durations of dual antiplatelet therapy (DAPT), which consists of aspirin and a P2Y12 inhibitor, in patients with concomitant chronic kidney disease (CKD) and acute coronary syndrome (ACS).
Materials and Methods
We searched PubMed, Cochrane, and Proquest from inception to January 2024 with the terms “acute coronary syndrome,” “chronic kidney disease,” and “dual antiplatelet therapy”, and a ten-year publication date restriction. We included observational studies that assessed the impact of DAPT on major adverse cardiovascular events (MACE) and safety outcomes, specifically major and minor bleeding events. The included studies involved patients with ACS diagnoses who also had CKD. Risk of bias assessment was assessed regarding selection, comparability, and outcome.
Results
We included eight studies involving 166,290 participants—six studies with a retrospective design and two with a prospective design. The quality of evidence was generally good. Six studies showed a significantly difference incidence of MACE in prolonged DAPT administration, in which five of them showed a decreased incidence. One study reported a higher two-year mortality in patients with CKD and prolonged DAPT compared to the general population. Bleeding risks were increased significantly in prolonged DAPT in two studies, five studies reported no significant difference in bleeding incidence, and one study reported a higher rate of adverse outcomes in lower estimated glomerular filtration rate compared to healthy patients when given DAPT for > 12 months.
Conclusion
Prolonged DAPT administration may decrease the risk of MACE with no increased risk in bleeding occurrences in patients with concomitant CKD and ACS.
Keywords
Acute coronary syndrome
Chronic kidney disease
Dual antiplatelet therapy
Efficacy
Safety
Introduction
The global prevalence of chronic kidney disease (CKD) is on the rise.1 Patients with CKD face a higher risk of coronary artery disease (CAD), acute coronary syndrome (ACS), and cardiovascular death. The combination of traditional cardiovascular risk factors and specific risk factors related to uremia speeds up the development of atherosclerosis, which accounts for the higher occurrence of major adverse cardiac events (the primary cause of death in individuals with CKD).2 This explains why 30% of ACS patients also have moderate to severe CKD.3 ACS includes non-ST segment elevation myocardial infarction (NSTEMI), unstable angina, ST segment elevation myocardial infarction (STEMI), and myocardial infarction with nonobstructive coronary arteries,4 in which percutaneous coronary intervention (PCI) for ACS is the standard treatment, followed by dual antiplatelet therapy (DAPT) (a combination of aspirin and a P2Y12 inhibitor). However, patients with CKD and concomitant ACS receive less intervention and have a worse prognosis compared to those with normal kidney function.5–7
Patients with CKD exhibit increased platelet reactivity, heightened inflammation, oxidative stress, endothelial dysfunction, and related health conditions predisposing them to thrombotic events.8 Conversely, they also face higher bleeding risk due to dysregulated platelet function and changes in drug metabolism.9 This poses a challenge for clinicians to balance the potential for thrombotic events against the risk of bleeding. There is also limited knowledge about the effectiveness and safety of DAPT in CKD patients, as they are often excluded from randomized controlled trials.
The recommended duration of DAPT regimens in ACS patients is generally 12 months.4,10 However, as CKD patients are exposed to a higher risk of thrombotic events and bleeding, it remains unclear whether they would benefit similarly from the standard treatment. The optimal duration of DAPT after PCI is still debatable. Guidelines generally recommend at least 12 months for ACS.11 In CKD patients, determining DAPT duration is more challenging due to the underrepresentation of their population in available research and increased bleeding risk due to platelet and endothelial dysfunction, abnormal coagulation, and reduced response to clopidogrel.
This systematic review evaluates the available researches of DAPT regimen in ACS patients with CKD to determine the efficacy and safety of different durations of DAPT.
Materials and Methods
We employed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) writing method as a guide for organizing the writing systematically. This study was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42024606690.
Eligibility Criteria
We aimed to evaluate the efficacy and safety of different durations of DAPT, which consists of aspirin and a P2Y12 inhibitor, in patients with CKD and ACS. Due to the need for a long follow-up to generate results of the outcome, this review focused on observational studies that assessed the impact of DAPT on major adverse cardiovascular events (MACE) and safety outcomes, specifically major and minor bleeding events. The included studies involved patients with ACS diagnoses, such as STEMI, NSTEMI, unstable angina pectoris (UAP), and myocardial infarction with nonobstructive coronary arteries, who also had CKD with a GFR of less than 60 mL/min/1.73 m2 for more than three months. We excluded studies that were not written in English or Bahasa Indonesia to maintain consistency and accessibility of data.
Search Strategy
We systematically searched PubMed, Cochrane, and Proquest from inception to January 2024. The terms “acute coronary syndrome,” “chronic kidney disease,” and “dual antiplatelet therapy” were utilized. We did not include words related to the outcomes of interest to increase search sensitivity. The search was restricted with a ten-year publication date filter. The complete search strategy is presented in Supplementary Material.
Study Selection and Data Extraction
The authors employed EndNote as a reference manager. We removed duplicate references and evaluated the titles and abstracts of the search results. Studies that did not meet the inclusion criteria were excluded. Subsequently, full texts of relevant studies were assessed, and those that fulfilled the eligibility criteria were included.
Next, the authors extracted pertinent information from the chosen studies, which encompassed methodological details and outcomes. In cases where complete data were unavailable, the authors reached out to the corresponding authors of the selected studies.
Two independent reviewers carried out the entire process of study selection and data extraction. Any discrepancies in study selection or data extraction were resolved through mutual agreement and consensus.
Risk of Bias and Quality Assessment
Two independent reviewers assessed the quality of the included studies using Newcastle-Ottawa Quality Assessment Form for Cohort Studies. Discrepancies in quality assessment were resolved through consensus or by a third reviewer.
Results
We identified 256 studies from our search. Eight studies were included—six with a retrospective and two with a prospective design—with a total of 166,290 participants. Seven studies reported MACE and bleeding with different DAPT duration as comparison and one study reported MACE as a solitary outcome. Figure 1 shows the flow diagram of the study selection.
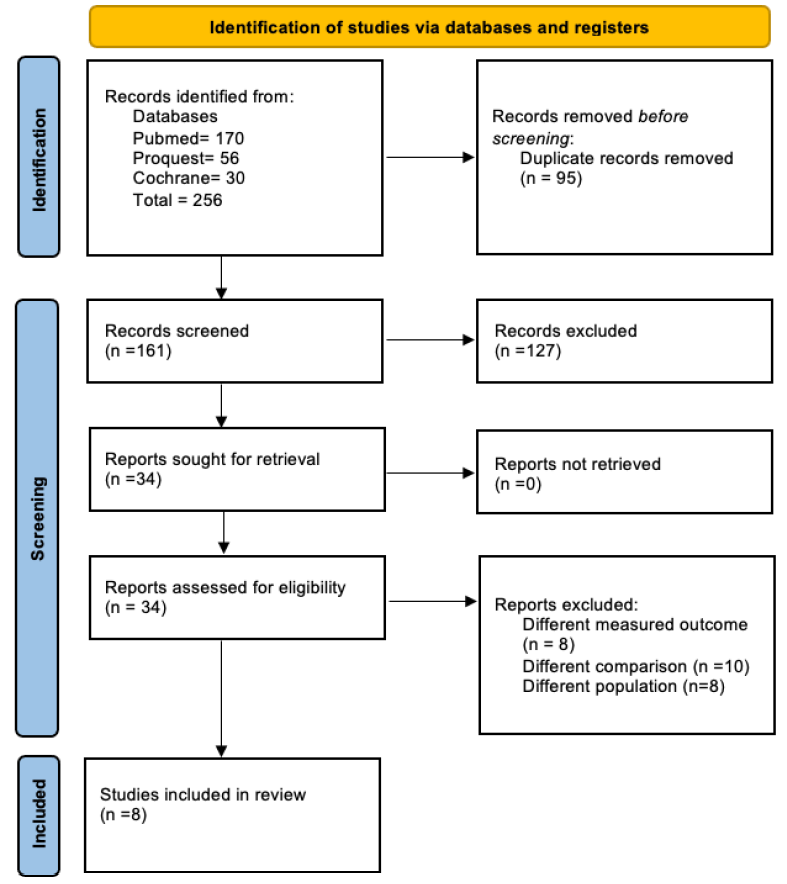
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
All studies provided clear descriptions of participants, protocols, and interventions. The majority of the participants were of the elderly population, ranging from 60 to 70 years old. Two studies included end-stage renal disease (ESRD) patients, one study did not specify CKD stage (but included dialysis patients), and three studies had a majority of mild to moderate CKD. P2Y12 inhibitors in this study used included clopidogrel, prasugrel, ticlopidine, and ticagrelor, the majority being clopidogrel. The follow-up points ranged from one month to seven years. Table 1 summarizes the main characteristics of the included studies.
| Study | Study design | Participants | DAPT and type of stent | Outcome | Duration of DAPT | Follow up time points |
|---|---|---|---|---|---|---|
| Siddiqi et al.14 | Retrospective cohort study |
23,042 patients receiving BMS or DES. CKD (eGFR < 60) occurred in 2,297 subjects receiving DES and 2,583 subjects receiving BMS. |
Clopidogrel; DES or BMS. | CKD patients with DAPT for more than 12 months had a lower risk of death or myocardial infarction (18% vs 24%, HR = 0.74; 95% CI 0.58–0.95) in patients that received DES. There was no increased risk of serious bleeding with prolonged clopidogrel in CKD patients receiving DES. | 12 months, ≤ 12 months | 12 months until 4 years |
| Asami et al.17 | Retrospective cohort study | 123 ESRD patients on hemodialysis who had undergone PCI with DES or BMS. | Clopidogrel and ticlopidine; DES or BMS. | Participants who received DAPT for 12 months had a higher incidence of MACE and no reduced stent thrombosis rate compared to patients who received DAPT for < 12 months. Bleeding events in patients who received DAPT for 12 months were 5.1 times greater than patients who received DAPT for < 12 months. | > 12 months, ≤ 12 months | 1 year and 7 years |
| Chen et al.15 | Retrospective cohort study | 2178 ESRD patients who were undergoing chronic dialysis and received a DES im- plantation. |
Clopidogrel; DES.
|
Patients undergoing hemodialysis who received prolonged DAPT after PCI with DES implantation (> 6 months) had lower risk of postprocedural death or myocardial infarction than those who discontinued DAPT. No association between prolonged DAPT and long-term major adverse cardiovascular events or bleeding complications were found. | < 6 months, ≥ 6 months | Death or until December 31, 2012 |
| Carrero et al.16 | Prospective cohort study | 36,001 patients with new onset ACS patients discharged with clopidogrel, where 4387 patients (12.2%) had an eGFR 45–60 mL/min/1.73 m2, 2127 (5.9%) had an eGFR 30–45 m/min per 1.73 m2, and 834 (2.3%) had an eGFR < 30 mL/min/1.73 m2. | Clopidogrel; DES or BMS. | CKD patients who continued DAPT after three months were associated with a lower incidence of death/ischemic stroke or reinfarction compared to DAPT stopped at three months. The risk of bleeding increases in the treatment group with longer DAPT duration. Overall, better outcomes were seen in the group that received longer DAPT in most eGFR strata, although there was no risk association in the eGFR of < 30 mL/min/1.73 m2 group. | 3 months, > 3 months | Day 111 and Day 365 |
| Park et al.3 | Retrospective cohort study | 5616 patients who had been on HD or PD and underwent DES implantation during the study period. | Clopidogrel, prasugrel, and ticagrelor; DS. | Continued DAPT was significantly associated with lower incidences during the entire follow-up period at all landmarks (12-month, 15-month, 18-month). The differences between groups in terms of major bleeding were not statistically significant. | 12, 15, and 18 months | 12, 15 and, 18 months |
| Kao et al.18 | Retrospective cohort study | 1899 patients who underwent PCI with CKD (ranging from low, moderate, high to very high risk). |
Clopidogrel and ticagrelor; DES or BMS.
|
There was no significant difference in MACE outcome between long-term (≥ 6 months) and short-term (< 6 months). Both groups had equal risk for TIMI bleeding. |
> 6 months, ≤ 6 months
|
6 months and 18 months |
| Huo et al.19 | Prospective cohort study | 23,490 patients who survived hospitalization for an ACS (STEMI, NSTEMI, or unstable angina) stratified by eGFR category. 41.9% patients had an eGFR ≥ 90v mL/min/1.73 m2, 41.4% had eGFR 60–89 mL/min/1.73 m2, 14.5% had eGFR 30–59 mL/min/1.73 m2, and 2.2% had eGFR < 3 mL/min/1.73 m2. | Unspecified. | Patients with lower eGFR had higher rates of adverse outcomes, including death, nonfatal MI, nonfatal stroke, and bleeding. Composite cardiovascular endpoint and death were higher in patients with poorer renal function in both adjusted and unadjusted analysis. | The average duration of DAPT ranged from 16.7 months in the eGFR < 30 mL/min/1.73 m2 category to 19.8 months in the eGFR ≥ 90 category (p < 0.0001). | 2 years |
| Kim et al.12 | Retrospective cohort study | 73941 patients (≥ 20 years old) who were treated with first- or new-generation DES with available kidney function information. | Clopidogrel, prasugrel, or ticagrelor; DES. | Prolonged DAPT was beneficial in decreasing the risk of cardiovascular death (pint = 0.02) or MI (pint = 0.02) with worsened renal dysfunction. Sensitivity analysis also showed that prolonged DAPT is associated with lower risk of composite ischemic event in patients with worsened renal function, irrespective of statistical models, criteria of MI, stent type, and population. No significant interaction between presence of CKD and bleeding risk (pint = 0.22). | 6–12 months, 12–24 months | N/A |
ACS: Acute coronary syndrome, eGFR: estimated glomerular filtration rate, STEMI: ST-Elevation Myocardial Infarction, NSTEMI: Non-ST Elevation Myocardial Infarction, N/A: Not available BMS: Bare metal stent, DES: Drug-eluting stents, MI: Myocardial infarction, PD: Peritoneal dialysis, HD: Hemodialysis, CKD: Chronic kidney disease, DAPT: Dual antiplatelet therapy, HR: Hazards ratio, CI: Confidence interval, ESRD: End-stage renal disease, MACE: Major cardiovascular adverse events, PCI: Primary coronary intervention, TIMI: Thrombolysis in myocardial infarction.
Risk of Bias
The risk of bias was assessed using the Newcastle-Ottawa Quality Assessment Form for Cohort Studies (NOS). All studies presented with a low risk of bias, which fulfilled the “good quality” criteria presented in NOS: three or four stars in selection domain, one or two stars in comparability domain, and two or three stars in outcome/exposure domain. All studies lacked the description and efforts to address the participants that were lost to follow-up. Two studies also did not describe whether the outcomes of interest were not present at the start of the study, while the exclusion criteria only excluded participants who were not able to tolerate antiplatelet therapy and who required withdrawal of antiplatelet therapy within three months for a planned surgery. Table 2 summarizes the overall risk of bias of all included studies.
| Author | Selection | Comparability | Outcome | Overall Risk of Bias# | |||||
|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | ||
| Carrero et al.16 | * | * | * | * | ** | * | * | - | Good |
| Kao et al.18 | * | * | * | * | ** | * | * | - | Good |
| Asami et al.17 | * | * | * | - | ** | * | * | - | Good |
| Huo et al.19 | * | * | * | * | ** | * | * | - | Good |
| Park et al.13 | * | * | * | * | ** | * | * | - | Good |
| Chen et al.15 | * | * | * | * | ** | * | * | - | Good |
| Siddiqi et al.14 | * | * | * | - | ** | * | * | * | Good |
| Kim et al.12 | * | * | * | * | ** | * | * | - | Good |
#Based on the thresholds for converting the Newcastle-Ottawa scales to AHRQ standards (good, fair, and poor), *Available in the study, **Study controls for age, sex, marital status, and other factors.
Study Synthesis
Five studies showed a decreased incidence of MACE in prolonged DAPT administration.12–16 One study reported an increased incidence of MACE in prolonged DAPT.17 One study showed no significant differences.18 One study reported a higher two-year mortality in patients with CKD and prolonged DAPT compared to the general population,19 bleeding risks were increased significantly in prolonged DAPT in two studies,16,17 five studies reported no significant difference in bleeding incidence,12–15,18 and one study reported a higher rate of adverse outcome in lower eGFR compared to healthy patients when given DAPT for > 12 months.19
Discussion
We found that prolonged DAPT administration was able to decrease the risk of MACE with no significant differences in bleeding occurrences. This may be due to the increased benefit of DAPT in CKD patients with a higher prevalence of CAD. Cardiovascular abnormalities in CKD are related to traditional and nontraditional CKD-related cardiovascular disease (CVD) risk factors. Other than the usual risk factors, such as diabetes and hypertension, other risk factors, such as mineral and bone disease abnormalities, inflammation, oxidative stress, anemia, uremia, type and frequency of dialysis, and dialysate composition, increased the chance of CVD.20 Patients with declining GFR have an increased risk of vascular calcifications, especially in subintima and media of large vessels, which contribute to all-cause and cardiovascular mortality.21
There were also several inconsistencies that need to be addressed. Kao et al.’s study reported similar risks of MACE in both long- and short-term DAPT administration.18 This may be due to the majority of the participants being in the low CKD risk group with eGFR > 60 and moderate albuminuria, in which DAPT duration does not impact the risk of clinical outcomes in healthier populations. The participants of this study were Asian, who have a higher proportion of inadequate CYP2C19 as a metabolizer, deeming clopidogrel regiments ineffective despite prolonged administration.22
We also discovered that three of the included studies had given clopidogrel to the majority of the study population, whereas only Asami et al. gave ticlopidine to their study population. The difference in the combination of regimens used could potentially lead to different outcomes due to variety in chemical structure.17 Being a first-generation thienopyridine, ticlopidine had several drawbacks. This includes thrombotic thrombocytopenic purpura, which could be the reason behind the higher incidence of MACE in the study.23 It was reported that the benefits from clopidogrel outweighs its risks when compared to ticlopidine. The study sample in Asami et al. is significantly smaller than other studies. This potentially increases the margin of error in the sample population, leading to study bias. Last, the follow-up duration in this study ran across seven years. Such long-term follow-up duration increases the risk of recall bias due to the inaccuracy of memory recollection by either the patients or their family members. Any unprecedented confounding factors that possibly emerge throughout the years could also alter the study results.24 Moreover, patients included in this study were ESRD patients, which may influence the prognosis of mortality. However, Huo et al.’s study19 classified their patients according to their eGFR levels. Hence, it further emphasized the fact that those with lower eGFR were associated with higher rates of MACE and bleeding within a two-year follow-up period. One reason that could lead to a poorer prognosis is due to the specific population in the study. In parallel with Kao et al.,18 the majority of the study population were Asian, which were claimed to have a higher risk of bleeding, such as intracranial hemorrhage, should they be under an anticoagulant treatment.
Strengths of our study include a comprehensive literature search with clear inclusion and exclusion criteria. We also assessed the risk of bias to determine the quality of evidence.
There are several limitations to our review. We only found eight studies despite our broad literature search, which we recommend searching in bigger databases to achieve higher comprehensibility. The available studies included were cohort studies, both prospective and retrospective. Due to the limited existing studies with randomized controlled trial designs, we recommend the randomized controlled trial design for future studies to investigate a clearer cause and effect relationship. The included studies had low risk of bias; however, there may still be the influence of complex comorbidities in patients with CKD to the results. All the studies also did not elucidate the loss to follow-up population, which may introduce bias as well.
Prolonged DAPT administration was found to decrease the risk of MACE without significant increases in bleeding incidents in patients with concomitant CKD and ACS. However, inconsistencies were noted, including a study suggesting similar MACE risks between short- and long-term DAPT, potentially influenced by participant characteristics and genetic factors affecting drug metabolism. Additionally, variations in antiplatelet regimens and study population characteristics were observed, impacting outcomes and introducing biases. Recommendations include conducting larger randomized controlled trials with clearer patient categorization and addressing follow-up population loss and comorbidity influences on results.
Acknowledgments
The authors would like to acknowledge the generous support from our university for the continuous encouragement, appreciation, and keen interest in our research.
Conflicts of interest
There are no conflicts of interest.
References
- KDIGO_2012_CKD_GL.pdf [Internet]. [Last accessed on 2023 Nov 3]. Available from: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf
- Acute coronary syndromes in chronic kidney disease: Clinical and therapeutic characteristics. Medicina (Kaunas). 2020;56:118.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Relation between renal function, presentation, use of therapies and in-hospital complications in acute coronary syndrome: Data from the SWEDEHEART register. J Intern Med. 2010;268:40-9.
- [CrossRef] [PubMed] [Google Scholar]
- 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC) Eur Heart J. 2023;44(38):3720-826.
- [CrossRef] [PubMed] [Google Scholar]
- Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201-8.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of chronic kidney disease on revascularization and outcomes in patients with st-elevation myocardial infarction. Am J Cardiol. 2021;150:15-23.
- [CrossRef] [PubMed] [Google Scholar]
- Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375:2073-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors for chronic kidney disease: An update. Kidney Int Suppl (2011). 2013;3:368-71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Platelet function in CKD: A systematic review and meta-analysis. J Am Soc Nephrol. 2021;32:1583-98.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dual antiplatelet therapy after percutaneous coronary intervention: Personalize the duration. Cleve Clin J Med. 2021;88:325-32.
- [CrossRef] [PubMed] [Google Scholar]
- Dual-antiplatelet therapy after percutaneous coronary intervention: How short is too short? J Am Heart Assoc. 2023;12:e028775.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Benefit and risk of prolonged dual antiplatelet therapy after drug-eluting stent implantation in patients with chronic kidney disease: A nationwide cohort study. Atherosclerosis. 2022;352:69-75.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcomes of prolonged dual antiplatelet therapy after coronary drug-eluting stent implantation in dialysis patients. Clin Kidney J. 2020;13:803-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Outcomes with prolonged clopidogrel therapy after coronary stenting in patients with chronic kidney disease. Heart. 2015;101:1569-76.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dual antiplatelet therapy and clinical outcomes after coronary drug-eluting stent implantation in patients on hemodialysis. Clin J Am Soc Nephrol. 2017;12:262-71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Long-term versus short-term dual antiplatelet therapy was similarly associated with a lower risk of death, stroke, or infarction in patients with acute coronary syndrome regardless of underlying kidney disease. Kidney Int. 2017;91:216-26.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of stent type and prolonged dual antiplatelet therapy on long-term clinical outcomes in hemodialysis patients with coronary artery disease. Cardiovasc Interv Ther. 2018;33:84-94.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of dual antiplatelet therapy after coronary stenting in patients with chronic kidney disease. PLoS One. 2021;16:e0255645.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Long-term antithrombotic therapy and clinical outcomes in patients with acute coronary syndrome and renal impairment: Insights from EPICOR and EPICOR Asia. Am J Cardiovasc Drugs. 2021;21:471-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic kidney disease and cardiovascular disease: Is there any relationship? Curr Cardiol Rev. 2019;15:55-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Atherosclerosis specific features in Chronic Kidney Disease (CKD) Biomedicines. 2022;10:2094.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pharmacogenomics in Asian subpopulations and impacts on commonly prescribed medications. Clin Transl Sci. 2020;13:861-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Challenges in conducting long-term outcomes studies in critical care. Curr Opin Crit Care. 2019;25:473-88.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








