Translate this page into:
Endothelial Dysfunction in Children with Frequently Relapsing and Steroid-Dependent Nephrotic Syndrome
Corresponding author: Abhijeet Saha, Division of Pediatric Nephrology, Department of Pediatrics, Lady Hardinge Medical College, New Delhi, India. E-mail: drabhijeetsaha@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Verma R, Deepthi B, Saha A, Bhattacharjee J. Endothelial Dysfunction in Children with Frequently Relapsing and Steroid-Dependent Nephrotic Syndrome. Indian J Nephrol. doi: 10.25259/ijn_568_23
Abstract
Background
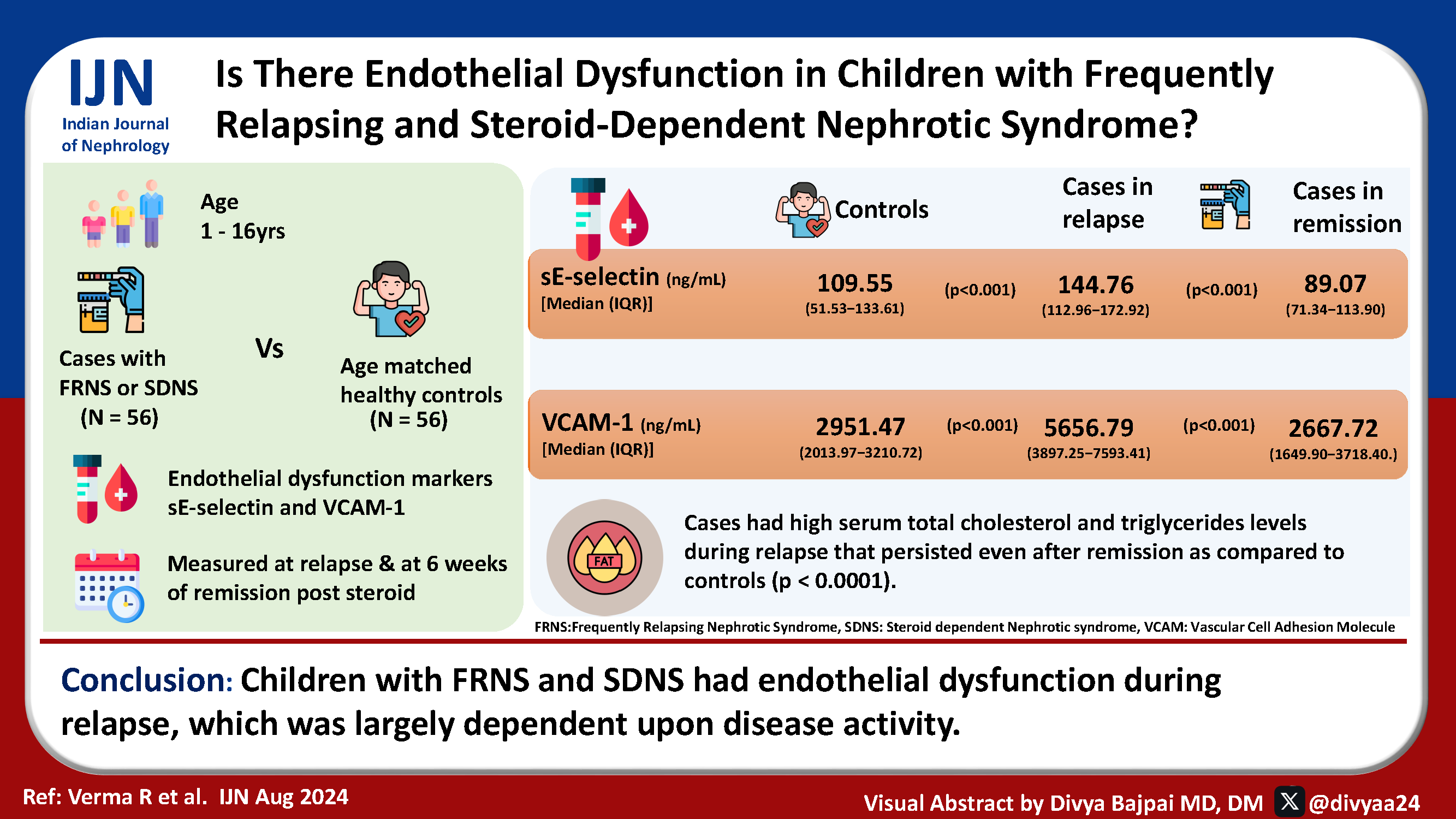
Children with frequently relapsing (FR) or steroid-dependent (SD) nephrotic syndrome (NS) show endothelial dysfunction and risk of endothelial injury during relapses, increasing the risk of accelerated atherosclerosis and adverse cardiovascular events. This study examines the plasma levels of markers of endothelial dysfunction [sE-selectin and vascular cell adhesion molecule-1 (VCAM-1)] in children aged 1–16 years with FRNS and SDNS in relapse.
Materials and Methods
Fifty-six children with FRNS and SDNS between 1 and 16 years were enrolled at the time of relapse and followed till six weeks of steroid-induced remission. Markers of endothelial dysfunction (sE-selectin and VCAM-1) in plasma were measured in these children and in an equal number of controls.
Results
Plasma sE-selectin and VCAM-1 levels were significantly raised during relapse, declined after six weeks of steroid-induced remission, and became comparable to controls (p < 0.0001). We found high serum total cholesterol and triglycerides levels during relapse that remained elevated even after steroid-induced remission as compared to controls (p < 0.0001). Raised levels of these markers confirm endothelial dysfunction in FRNS and SDNS patients.
Conclusion
Children with FRNS and SDNS had endothelial dysfunction during relapse, which was largely dependent upon disease activity.
Keywords
Endothelial dysfunction
sE-selectin
VCAM-1
FRNS
SDNS
Introduction
The activated endothelium expresses various adhesion molecules and selectins, whose soluble forms are detected in the plasma. Leucocytes bind to the activated endothelium via the cellular adhesion molecules (CAMs), including the selectins and immunoglobulin superfamily [vascular cell adhesion molecule (VCAM-1), immunoglobulin cell adhesion molecule (IgCAM-1)]. There is increased expression of these molecules under the influence of atherogenic factors, facilitates rolling, activation, tight adhesion, and transendothelial migration of leucocytes into the intima, where they initiate inflammatory process.1,2 Further stimulation of these pathways leads to the initiation of atherosclerosis. CAMs are potential and key components of the thrombotic process.3
The inflammatory genesis of atherosclerosis with crucial role of adhesion molecules leads us to speculate that assessing adhesion molecules may accurately predict the risk of endothelial dysfunction and hence vascular complications in frequently relapsing nephrotic syndrome (FRNS)/steroid-dependent nephrotic syndrome (SDNS) patients. The studies on the evaluation of role of adhesion molecules in endothelial dysfunction and its association with traditional risk factors in FRNS/SDNS are scarce. The primary aim is to study plasma levels of markers of endothelial dysfunction (sE-Selectin and sVCAM-1) in children aged 1–16yrs with FRNS and SDNS in relapse.
Materials and Methods
This cross-sectional study was conducted in the Departments of Pediatrics and Biochemistry at Lady Hardinge Medical College and Kalawati Saran Children’s Hospital, New Delhi, from November 2016 to April 2018 after obtaining Institute ethics committee approval. A written informed consent was obtained from parents or guardians of all children enrolled in the study. The primary objective was to study the plasma levels of markers of endothelial dysfunction (sE-Selectin and sVCAM-1) in children with FRNS and SDNS during relapse as compared to controls. We also evaluated levels of these markers after steroid induced complete remission at six weeks. Children aged 1–16 years with idiopathic FRNS and SDNS in relapse were included. Children with secondary nephrotic syndrome (NS) (e.g., lupus nephritis, IgA nephropathy, Henoch–Schönlein purpura nephritis), those with signs of thromboembolic complications, or children on drugs affecting endothelial functions (statins), Children with preexisting hypertension and diabetes mellitus or with known bleeding diathesis were excluded. Children previously enrolled in the study were not re-enrolled during further relapse. Age-matched healthy, nonobese (WHO standards, 20074), nonhypertensive children were taken as controls.5 NS was defined by the presence of nephrotic range proteinuria (spot urinary protein to creatinine ratio more than 2 or urinary dipstick +3/+4 for three consecutive days or 24 hour protein >40 mg/m2/hr), hypoalbuminemia (serum albumin <2.5g/dl), and serum cholesterol >200mg/dL. NS was classified, investigated, and managed as per the Indian Society of Pediatric Nephrology group guidelines.6 SDNS was defined as two consecutive relapses when on alternate day steroids or within 14 days of stopping it. FRNS was defined as two or more relapses within six months of initial episode or more than three relapses within any 12-month period.
Sample size estimation
The true difference in the cases and control means for sE-selectin in a previous study was 18.12ng/mL and pooled standard deviation was 33.88. A sample size of 56 in each group was required to achieve significant results with 5% and study power of 80% for sE-selectin. For VCAM-1, the true difference in the cases and controls means was 683.09ng/mL and the pooled standard deviation was 363.89 and a sample size of six was needed. We used the larger sample of the two and included 56 cases and 56 controls to reject the null hypothesis with the power of 0.8 and alpha error of 0.05.
Sample collection, storage, measurements
Three milliliter blood was collected by venipuncture and serum separated by centrifugation at 2800 xg for 10 minutes. The samples were stored at ‒200C until analysis. Samples were collected at relapse and at six weeks of prednisolone-induced remission.
Measurement of endothelial dysfunction markers
VCAM-1 and sE-selectin, were measured by ELISA using kits by BioVendor Laboratory Medicine Inc., Karasek, Czech Republic. The measurement was done on Tecan Eliza machine present at the Department of Biochemistry, Lady Hardinge Medical College.
Statistical analysis
Data were analyzed using SPSS version 20. Continuous variables were tested by Mann Whitney U test and dichotomous variables were tested by Fisher’s exact. Correlation statistics was done among variables of interest and Spearman’s correlation coefficient was reported. Less than 0.05 of P value was considered as significant.
Results
A total of 112 children (56 cases and 56 controls) were included. Four patients were excluded because of noncompliance to drug therapy and two patients were lost to follow-up after enrolment. The cases and controls were matched for age and gender [Tables 1, 2, and 3]. Of the study group, 49 were frequent relapsers and 7 were steroid dependent. The mean age of patients was 6.84 years. The difference in weight and body mass index (BMI) in the case relapse group was significant compared to controls. The cases relapse group had abnormal lipid profile during both relapse and remission. The cases in relapse also had significantly high urine protein/creatinine ratio (Up:Uc) and low serum albumin. Out of the 56 cases in relapse, 16 (28.5%) had normal blood pressure, 19 (33.9%) had elevated blood pressure, and 15 (26.7%) had stage 1 and 6 (10.7%) had stage 2 hypertension, respectively. During remission, 22 (39.2%) had normal blood pressure, 27 (48.2%) had elevated blood pressure, and 6 (10.7%) had stage 1 and 1 (0.1%) had stage 2 hypertension. Hence, 28.5% patients were normotensive and 71.4% patients had high blood pressure readings in relapse. Similarly, 39.2% patients were normotensive and 59% patients had high blood pressure readings in remission. None of the controls were hypertensive. The plasma levels of sE-selectin and VCAM-1 were significantly higher in relapse compared to both controls and in remission [Table 4]. The plasma levels of VCAM-1 showed a significant correlation with total triglyceride, while sE-selectin showed a significant negative correlation with serum albumin levels [Table 5].
| Parameter |
Cases relapse (mean ± SD) (n = 56) |
Controls (mean ± SD) (n = 56) |
p-value |
|---|---|---|---|
| Age (years) | 6.84 ± 2.98 | 6.80 ± 3.37 | 0.941 |
| Body mass index (kg/m2) | 17.27 ± 2.37 | 15.42 ± 2.45 | <0.001 |
| Serum creatinine (mg/dl) | 0.34 ± 0.09 | 0.3 ± 0.1 | 0.0781 |
| eGFR (ml/min/1.73 m2) | 142.06 ± 43.03 | 163.22 ± 51.1 | 0.0195 |
| Total protein (g/dl) | 4.54 ± 0.79 | 7.01 ± 0.46 | <0.0001 |
| S. Albumin (g/dl) | 2.0 ± 0.69 | 4.43 ± 0.37 | <0.0001 |
| Urine protein creatinine ratio | 5.97 ± 5.81 | 0.13 ± 0.05 | <0.0001 |
| Total cholesterol (mg/dl) | 461.01 ± 159.65 | 154.03 ± 22.99 | <0.0001 |
| Triglycerides (mg/dl) | 372.23 ± 184.03 | 84.91 ± 18.46 | <0.0001 |
eGFR: estimated glomerular filtration rate.
| Parameter |
Cases remission (mean ± SD) (n = 56) |
Controls (mean ± SD) (n = 56) |
p-value |
|---|---|---|---|
| Age (years) | 6.84 ± 2.98 | 6.80 ± 3.37 | 0.941 |
| Body mass index (kg/m2) | 16.30 ± 2.39 | 15.42 ± 2.45 | 0.0569 |
| Serum creatinine (mg/dl) | 0.32 ± 0.09 | 0.3 ± 0.1 | 0.2040 |
| eGFR (ml/min/1.73 m2) | 155.33 ± 40.55 | 163.22 ± 51.1 | 0.3676 |
| Total protein (g/dl) | 5.21 ± 0.62 | 7.01 ± 0.46 | <0.0001 |
| Serum albumin (g/dl) | 2.53 ± 0.47 | 4.43 ± 0.37 | <0.0001 |
| Urine protein creatinine ratio | 0.20 ± 0.11 | 0.13 ± 0.05 | 0.0026 |
| Total cholesterol (mg/dl) | 344 ± 90.65 | 154.03 ± 22.99 | <0.0001 |
| Triglycerides (mg/dl) | 260.17 ± 115.56 | 84.91 ± 18.46 | <0.0001 |
eGFR: estimated glomerular filtration rate.
| Parameter |
Cases relapse (mean ± SD) (n = 56) |
Cases remission (mean ± SD) (n = 56) |
p-value |
|---|---|---|---|
| Age (years) | 6.84 ± 2.98 | 6.84 ± 2.98 | – |
| Body mass index (kg/m2) | 17.27 ± 2.37 | 16.30 ± 2.39 | 0.0332 |
| Serum creatinine (mg/dl) | 0.34 ± 0.09 | 0.32 ± 0.09 | 0.2422 |
| eGFR (ml/min/1.73 m2) | 142.06 ± 43.03 | 155.33 ± 40.55 | 0.0959 |
| Total protein (g/dl) | 4.54 ± 0.79 | 5.21 ± 0.62 | <0.0001 |
| Serum albumin (g/dl) | 2.0 ± 0.69 | 2.53 ± 0.47 | <0.0001 |
| Urine protein creatinine ratio | 5.97 ± 5.81 | 0.20 ± 0.11 | <0.0001 |
| Total cholesterol (mg/dl) | 461.01 ± 159.65 | 344 ± 90.65 | <0.0001 |
| Triglycerides (mg/dl) | 372.23 ± 184.03 | 260.17 ± 115.56 | 0.0002 |
eGFR: estimated glomerular filtration rate.
| Markers |
Cases in relapse (n = 56) |
Cases in remission (n = 56) |
Controls (n = 56) |
p-value |
|---|---|---|---|---|
| sE-selectin (ng/mL) | 144.76 | 89.07 | 109.55 | *<0.001 |
| [Median (IQR)] | (112.96‒172.92) | (71.34‒113.90) | (51.53‒133.61) | **<0.001 |
| ***0.535 | ||||
| VCAM-1 (ng/mL) | 5656.79 | 2667.72 | 2951.47 | *<0.001 |
| [Median (IQR)] | (3897.25‒7593.41) | (1649.9‒3718.40) | (2013.97‒3210.72) | **<0.001 |
| ***0.617 |
IQR: interquartile range, VCAM: Vascular cell adhesion molecule. *Statistical difference of cases in relapse as compared to cases in remission and Wilcoxon’s rank sum test applied. **Statistical difference of cases in relapse as compared to controls and Mann-Whitney test applied. ***Statistical difference of cases in remission as compared to controls and Mann-Whitney test applied, sE: Soluble E Selectin, FRNS: Frequently relapsing nephrotic syndrome, SDNS: Steroid dependent nephrotic syndrome.
| Parameters | sE-selectin | VCAM-1 | |
|---|---|---|---|
| Total cholesterol | r value | ‒0.06 | ‒0.06 |
| p value | 0.63 | 0.66 | |
| Serum Albumin | r value | ‒0.32 | ‒0.06 |
| p value | 0.01 | 0.66 | |
| Total triglyceride | r value | ‒0.06 | 0.30 |
| p value | 0.66 | 0.02 | |
| Spot Up:Uc ratio | r value | 0.23 | 0.06 |
| p value | 0.08 | 0.64 | |
Test name: Spearman’s correlation. r-spearman’s rho, Up:Uc ratio: urine protein: urine creatinine ratio, VCAM: vascular cell adhesion molecule, sE: Soluble E Selectin.
Discussion
We found that plasma sE-selectin and VCAM-1 levels were significantly raised in cases in relapse compared to remission and controls. Raised levels of these markers confirm endothelial dysfunction in FRNS and SDNS patients in relapse. The levels decreased in remission.
Endothelial cells reacting to oxidized low-density lipoprotein (LDL) in vitro releases a variety of molecules, including Von Willebrand factor (vWf), sVCAM-1, and sE-selectin, which are considered as markers of endothelial dysfunction or activation.7,8 Selectins arbitrate the first step of rolling of leucocytes in the complex inflammatory adhesion cascade, allowing leucocytes to interact with endothelial cells. These interactions lead to consequent signaling events through selectin ligands, which initiate atherosclerotic changes and cardiovascular diseases.9,10 sE-selectin seems to be important in the detection of the destruction of the endothelium in coronary heart disease.11 High concentrations of sE-selectin are distinctive for patients with moderate coronary heart disease, whereas for patients with more advanced disease, a rise in ICAM-1 predominates.1 Demerath et al., in their study, concluded that elevated serum concentrations of E-selectin and other adhesion molecules may be independent risk factors for atherosclerosis and cardiovascular diseases. E-selectin, unlike other adhesion molecules, is synthesized only by endothelium, with activating signals from IL-6 and TNF-alpha.12 Metabolic risk factors interact with inflammatory and immune mechanisms with resultant coronary artery disease.13
The plausible reason for induction of these signaling pathways in nephrotic patients is also attributed to the presence of hyperlipidemia. In our index study, derangements of lipid metabolism were prominent in relapse/active stage, adding further evidence to the hypothesis that lipid derangements cause endothelial activation and dysfunction. Many complications of NS like increased risk of atherosclerosis and thromboembolism can be attributed to dyslipidemia, commonly in those who don’t achieve complete remission. Dyslipidemia also leads to the development of glomerular injury.14 In nephrotic state, mechanisms like hypoalbuminemia, dyslipidemia, oxidative stress, and insulin resistance can cause endothelial injury.15,16
Various determinants of endothelial dysfunction have been described in literature, including markers like soluble thrombomodulin (sTM), vWF, plasminogen activator inhibitor-1 (PAI-1), tissue plasminogen activator (t-PA), plasma free homocysteine levels, endothelin 1, and CD 146.17–19
Plasma levels of CAMs are associated with many conventional and promising cardiovascular risk factors.20–22 We found a positive correlation of VCAM-1 with total triglyceride levels. sE-selectin levels had a significant negative correlation with serum albumin levels. In agreement with our findings, sVCAM had significant positive correlations with cholesterol levels while sE-selectin showed no statistically significant correlation with conventional risk factors in adult onset minimal change disease (MCD) patients.22 The study in focal segmental glomerulosclerosis (FSGS) patients also showed significant correlations of circulating endothelial cells (CECs) with cholesterol and sVCAM-1 with serum albumin.23 Traditionally, hyperlipidemia and hypoalbuminemia have shown to play an important role in pathogenesis of endothelial dysfunction and injury.15 Our study adds to this evidence. Inconsistent correlation between endothelial markers and traditional risk factors in literature leads to speculate that adhesion molecules may be independent risk factors of endothelial dysfunction even in the absence of traditional cardiovascular risk factors.24
Our results were comparable to the findings of Yildiz et al., who studied soluble adhesion molecule (sE-Selectin levels) in 48 children with steroid sensitive nephrotic syndrome (SSNS) versus 19 healthy controls. The serum levels of sE-selectin were higher in active stage compared to remission and controls.21
Numerous studies have demonstrated that children with NS have endothelial dysfunction. The American Heart Association Paediatric Consensus Guidelines has selected NS as a “special risk category” for accelerated atherosclerosis, supported by evidence of vascular dysfunction verified by noninvasive means.25 A published study in patients with primary FSGS also depicts that biomarkers of endothelial dysfunction (CECs, sTM, vWf, sVCAM-1, and sE-selectin) were significantly higher during active stage when compared with controls. Their work also highlights the key observations of increased incidence of thromboembolisms in patients with higher circulating endothelial markers, signifying the role of endothelial injury in vascular complications like thrombosis.23 The incidence of thromboembolisms spans from 2% in children to as high as 42% in adults with NS.26,16 A recent study aimed to evaluate sVCAM-1 and sE-selectin in adult-onset MCD. They concluded that patients with active MCD had increased levels of soluble adhesion molecules, which subsequently decreased as the disease went into remission, but the increase in sVCAM-1 persisted even after complete remission for six months.22 Our index study demonstrated that both the biomarkers attained normal levels during remission with comparable values to controls.
Considering the paucity of data on adhesion molecules in FRNS/SDNS children, this study adds to the existing evidence. We evaluated the levels at two points in time in the same patients (relapse and remission), giving insight that the levels of these biomarkers normalize during remission. Limitations of our study include small population size, single center study, and unavailability of urinary levels of these biomarkers.
Conclusion
Children with FRNS/SDNS had endothelial dysfunction during relapse, which was largely dependent upon disease activity.
Conflicts of interest
There are no conflicts of interest.
References
- Soluble ICAM-1 and VCAM-1 as markers of endothelial activation. Scand J Immunol. 2008;67:523-31.
- [CrossRef] [PubMed] [Google Scholar]
- Soluble adhesion molecules (sICAM-1, sVCAM-1) and selectins (sE selectin, sP selectin, sL selectin) levels in children and adolescents with obesity, hypertension, and diabetes. Metabolism. 2005;54:1020-6.
- [CrossRef] [PubMed] [Google Scholar]
- Venous and arterial thrombosis: A continuous spectrum of the same disease? Eur Heart J. 2005;26:3-4.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904.
- [CrossRef] [PubMed] [Google Scholar]
- Revised guidelines for management of steroid-sensitive nephrotic syndrome. Indian J Nephrol. 2008;18:31-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endothelial cell activation, injury, damage and dysfunction: Separate entities or mutual terms? Blood Coagul Fibrinolysis Int J Haemost Thromb. 2000;11:623-30.
- [CrossRef] [Google Scholar]
- Assessment of endothelial damage in atherosclerotic vascular disease by quantification of circulating endothelial cells. Relationship with von Willebrand factor and tissue factor. Eur Heart J. 2004;25:371-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cellular adhesion molecules and cardiovascular disease. Part I. Their expression and role in atherogenesis. Intern Med J. 2003;33:380-6.
- [CrossRef] [PubMed] [Google Scholar]
- Selectins: Initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107:331-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Circulating E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in men with coronary artery disease assessed by angiography and disturbances of carbohydrate metabolism. Metabolism. 2002;51:733-6.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship of soluble ICAM-1, VCAM-1, P-selectin and E-selectin to cardiovascular disease risk factors in healthy men and women. Ann Hum Biol. 2001;28:664-78.
- [CrossRef] [PubMed] [Google Scholar]
- Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685-95.
- [CrossRef] [PubMed] [Google Scholar]
- Dyslipidaemia in nephrotic syndrome: Mechanisms and treatment. Nat Rev Nephrol. 2018;14:57-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endothelial function in proteinuric renal disease. Kidney Int Suppl. 1999;71:S57-61.
- [CrossRef] [PubMed] [Google Scholar]
- Insulin resistance, dyslipidaemia, inflammation and endothelial function in nephrotic syndrome. Nephrol Dial Transplant. 2002;17:2220-5.
- [CrossRef] [PubMed] [Google Scholar]
- Endothelial dysfunction in children with frequently relapsing and steroid-resistant nephrotic syndrome. Asian J Pediatr Nephrol. 2020;3:4.
- [CrossRef] [Google Scholar]
- Endothelial dysfuntion in children with idiopathic nephrotic syndrome. Atherosclerosis. 2014;233:704-6.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma free homocysteine levels in children with idiopathic nephrotic syndrome. Indian J Nephrol. 2019;29:186-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Is there a link between CD146, a novel adhesion molecule and other markers of endothelial dysfunction in nephrotic syndrome and continuous ambulatory peritoneal dialysis? Thromb Res. 2005;115:19-24.
- [CrossRef] [PubMed] [Google Scholar]
- CD19 + CD23+ B cells, CD4 + CD25+ T cells, E-selectin and interleukin-12 levels in children with steroid sensitive nephrotic syndrome. Ital J Pediatr. 2013;39:42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Soluble vascular cell adhesion molecule-1 is associated with disease activity in adult-onset minimal change disease. Am J Med Sci. 2019;357:311-5.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers of endothelial dysfunction in patients with primary focal segmental glomerulosclerosis. Nephrology. 2012;17:338-45.
- [CrossRef] [PubMed] [Google Scholar]
- Markers of endothelial dysfunction in children with idiopathic nephrotic syndrome. Am J Nephrol. 2008;28:197-202.
- [CrossRef] [PubMed] [Google Scholar]
- Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics. 2011;128:S213-56.
- [CrossRef] [PubMed] [Google Scholar]
- High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: Results from a large retrospective cohort study. Circulation. 2008;117:224-30.
- [CrossRef] [PubMed] [Google Scholar]








