Translate this page into:
Endovascular Embolization in Problematic Hemodialysis Arteriovenous Fistulas: A Nonsurgical Technique
Address for correspondence: Dr. Raghunandan Prasad, Department of Radio Diagnosis, Sanjay Gandhi Post graduate Institute of Medical Sciences, Lucknow - 226 014, Uttar Pradesh, India. E-mail: coolraghu@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
In the past, surgical techniques were considered gold standard practice for obliterating the accessory veins, reducing the flow across the high flowing arteriovenous fistulas (AVFs), or for closing the problematic hemodialysis AVFs. However, recently endovascular embolization has emerged as a safe and cost-effective alternative to these surgical techniques. In this study, technical and clinical success, and safety of endovascular embolization have been evaluated for accessory vein obliteration, flow reduction, and fistula closure in problematic AVFs using various embolizing agents.
Methods:
This is a retrospective study of patients with problematic hemodialysis AVFs, who underwent endovascular embolization for accessory vein obliteration, flow reduction, and AVF closure at our center from February 2017 to January 2019 with various embolic agents like vascular plugs (VP), thrombin, coils, and glue. Follow-up was done at 1 week, 3 months, 6 months, and annually thereafter.
Results:
In this study 30 patients with problematic hemodialysis AVFs [Left brachiocephalic fistula (BCF) (n = 22), right BCF (n = 4), and left radiocephalic fistula (RCF) (n = 4)], underwent endovascular embolization for accessory vein obliteration (n = 6), flow reduction (n = 3), and AVF closure (n = 21). Of the 6 patients undergoing embolization for obliteration of accessory collateral, 4 patients had nonmaturing AVFs and 2 patients had symptoms of venous hypertension (VH). Post embolization, all 4 AVFs matured over a month and symptoms of VH completely resolved within a week. Three patients who underwent embolization for flow reduction had patent AVF (on doppler) post procedure and they achieved adequate flow during dialysis with complete resolution of symptoms of VH. Out of 21 patients, who underwent endovascular closure, complete AVF thrombosis was seen in 18 patients only with the use of VP, while 4 patients required additional procedure to achieve complete thrombosis of AVF.
Conclusion:
Endovascular embolization in problematic hemodialysis AVF is a safe and cost-effective alternative to open surgical methods and vascular plug could be embolic agent of choice for AVF closure.
Keywords
AV fistula
endovascular closure
endovascular plug
hemodialysis access
Introduction
As per national kidney foundation-Kidney Disease Outcome Quality Initiative (NKF-KDOQI) clinical practice guidelines, native arteriovenous fistulas (AVFs) remain the preferred vascular access to deliver the hemodialysis for end-stage renal disease (ESRD) patients because of its longer survival and lower infection rates.[1] However, AVFs are also associated with various complications like primary failure/nonmaturation, venous hypertension (VH), aneurysmal dilatation of draining vein, dialysis associated steal syndrome (DASS), and hyperdynamic heart failure.[2345] Primary failure or nonmaturation of AVF is most commonly associated with venous stenosis and accessory draining veins (ADVs) competing for blood flow in up to 40% of the cases of primary AVF failures.[67] Endovascular embolization of ADVs has emerged as an effective alternative to surgical treatment.[7]
Features of VH along with aneurysmal dilatation of draining vein are frequently associated with central vein stenosis, and less commonly with peripheral vein stenosis. In the latter situation, they are usually associated with reverse flowing venous collateral.[8] Majority of central and peripheral vein stenoses are amenable to treatment by surgical or endovascular techniques. However, in case of failure by both methods, fistula closure with alternative route of dialysis is the only treatment option. Patients with symptoms of VH present with extensive swelling of ipsilateral extremity and chest along with skin changes (skin discoloration, puckering, and thickening): Posing a surgical challenge to detect and ligate the fistula.[9] In situations where a precious high flowing problematic AVF cannot be sacrificed, flow reduction procedures can be used as they preserve access functionality.[10]
With advances in interventional radiology, endovascular methods of AVF ligation and flow reduction have promising results.[311] Embolizing agents like coils, detachable balloons, vascular plug (VP) device, thrombin, and n-butyl 2-cyanoacrylate (NBCA) glue have been used to occlude the AVF.[1213141516] VP devices like Amplatzer vascular plug (AVP) (St. Jude Medical, Inc., Minnesota, USA) or Cera- series of plugs (Lifetech Scientific Corp., China) are the newer embolizing agents.
We report our experience with the endovascular embolization of AVF for the purpose of accessory vein or collateral vein obliteration, flow reduction, and fistula closure using VP, coils, and thrombin/NBCA glue as embolizing agents.
Methods
This is a retrospective study of patients who underwent percutaneous endovascular embolization of AVF in a tertiary hospital from 1st February, 2017 to 31st January 2019.
The indications for AVF embolization included (i) fistula closure for patients presenting with intractable arm edema due to chronic central vein occlusion (CVO), (ii) fistula flow reduction in patients with aneurysmal dilatation of draining vein with or without CVO, and (iii) flow diversion by embolizing accessory vein/collateral in patients with nonmaturing AVF to help in fistula maturation or to reduce symptoms of VH by embolizing reverse flowing vein/collateral.
Embolizing agent and technique
In all patients of AVF closure, flow reduction and in two patient of flow diversion, VP was the embolizing agent of choice. While in four cases (of flow diversion) platinum coils were used, one patient underwent percutaneous NBCA glue and four patients had percutaneous thrombin embolization in addition to VP. A 20%–50% oversizing of the VP was done in reference to the adjacent vein diameter to avoid the risk of plug migration (i.e. 50% for AVF closure and 20% for flow reduction).
In all cases of fistula closure and flow reduction, a retrograde puncture of the out-flow vein approximately 4 to 6 cm away from the anastomotic site was done and access secured with a femoral/radial sheath (6-8F Cook/Terumo). Venous puncture site was chosen close to the anastomotic site to allow the deployment of VP via the introducer sheath, thus minimizing the additional cost of long sheath. The anastomotic segment was negotiated with the 0.035-inch Glide-Wire (Terumo, Europe) and a 5-Fr diagnostic catheter (Kumpe; Cook, Bloomington, IN, UA) and the diagnostic catheter was placed at the juxta-anastomotic arterial side. Diagnostic contrast fistulography was obtained to study anatomy, vein size, and to look for any collateral or retrograde flowing vein apart from the main outflow vein. Under road map/ultrasound (USG) guidance, the access sheath was advanced over the wire, just proximal to the anastomotic site in the AVF. VP was loaded into the sheath and deployed at the juxta-anastomotic venous site. VP deployment was done by steadily withdrawing the sheath over the plug while holding the delivery cable steady in position. The VP expanded and adhered to the vessel wall with an outward radial force. Once deployment was complete, the VP was detached by rotating the delivery cable counterclockwise. VP could be withdrawn and repositioned several times prior to final detachment, but a precise placement was essential as deployment distal to the anastomosis would result in a patent vein stump with incomplete closure and extension of VP into the artery could cause thrombosis of the artery. Doppler evaluation was done post deployment to look for flow in the venous segment.
In patients undergoing flow reduction, pre and post procedure per minute flow volume (milliliters per minute) was measured using USG as per the formula: 3.14 × square of the radius in centimeters × mean peak systolic velocity (PSV) (in cm/s) × 60. Mean flow velocity and radius of vein was measured by USG.
All procedures were done under local anesthesia on daycare basis. The patients were monitored for 4 h post procedure and then discharged. All the patients were followed up at 1 week, 3 months, 6 months, and annually thereafter. During the follow-up, the patients were assessed for resolution of clinical symptoms and physical examination of extremity along with USG color Doppler to look for thrombosis of the fistula, location of VP, any collateral or recanalization, and examination of arterial flow.
Results
Patient population
Out of the total 30 patients, who underwent endovascular embolization, 13 were male and 17 were female with a mean age of 53 years (range: 28 years–89 years). 26 patients had brachiocephalic fistula (BCF: 22 in left hand and 4 in right hand) and four patients had Radiocephalic fistula (RCF) in the left hand. The mean age of the AVFs was 25 months (range: 8 months–66 months).
The indications for AVF embolization included (i) fistula closure (n = 21) for patients presenting with intractable arm edema [Figure 1] due to chronic central vein occlusion (CVO), (ii) fistula flow reduction (n = 3) in patients with aneurysmal dilatation of draining vein with or without CVO, and (iii) flow diversion (n = 6) by embolizing accessory vein/collateral in patients with nonmaturing AVF to help in fistula maturation or to reduce symptoms of VH by embolizing reverse flowing vein/collateral. Among the six patients who underwent embolization for flow diversion, two patients had symptoms of VH in hand due to reverse flow in basilic vein in forearm. Rest of the four patients had nonmaturing AVFs with poor flow in outflow vein due to steal by accessory veins.
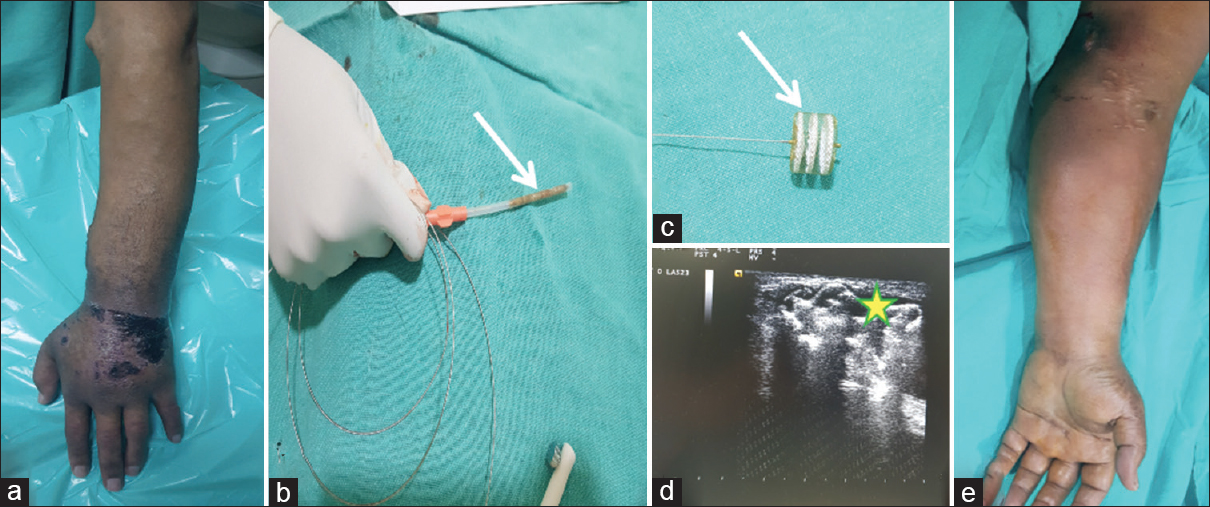
- 56-year-old male with left brachiocephalic AVF with intractable arm edema of the left hand with skin ulceration and discoloration (a), CERA vascular plug (VP) loaded into the sheath (white arrow, b), VP in its expanded form (white arrow, c), ultrasound (USG) image shows VP (yellow star*) in situ at the juxta-anastomotic venous site (d). 1-month follow-up shows resolution of the edema and clinical improvement in the left hand of the patient (e)
Among the three patients who underwent embolization for flow reduction, two patients were having very high flow (mean flow volume: 640 ml/min) across the AVF with aneurysmal dilatation of cephalic vein in arm and one was a case of hyperfunctioning left BCF (flow volume: 660 ml/min) with chronic occlusion of left brachiocephalic vein (which could not be recanalized by endovascular approach) causing severe arm, chest, and breast edema.
Out of the (n = 21) cases who underwent endovascular closure of AVF, seven patients had a well-functioning renal allograft (mean duration: 4 years), while 13 patients had alternative access for dialysis (two patients with tunneled central venous catheter [CVCs], 10 patients with right BCF, and one on continuous ambulatory peritoneal dialysis [CAPD]).
The indications for closing the fistula in 18 patients were symptoms of chronic VH secondary to central venous occlusion (CVO) which could not be recanalized by endovascular approach (average number of interventions: 2.6) [Figure 2]. In the remaining two patients (Case no 1 and 6), the indication for AVF closer was aneurysmal dilatation of cephalic vein in the arm. Indication for AVF embolization in one patient (Case no 7) was DASS with associated chronic radial artery occlusion.
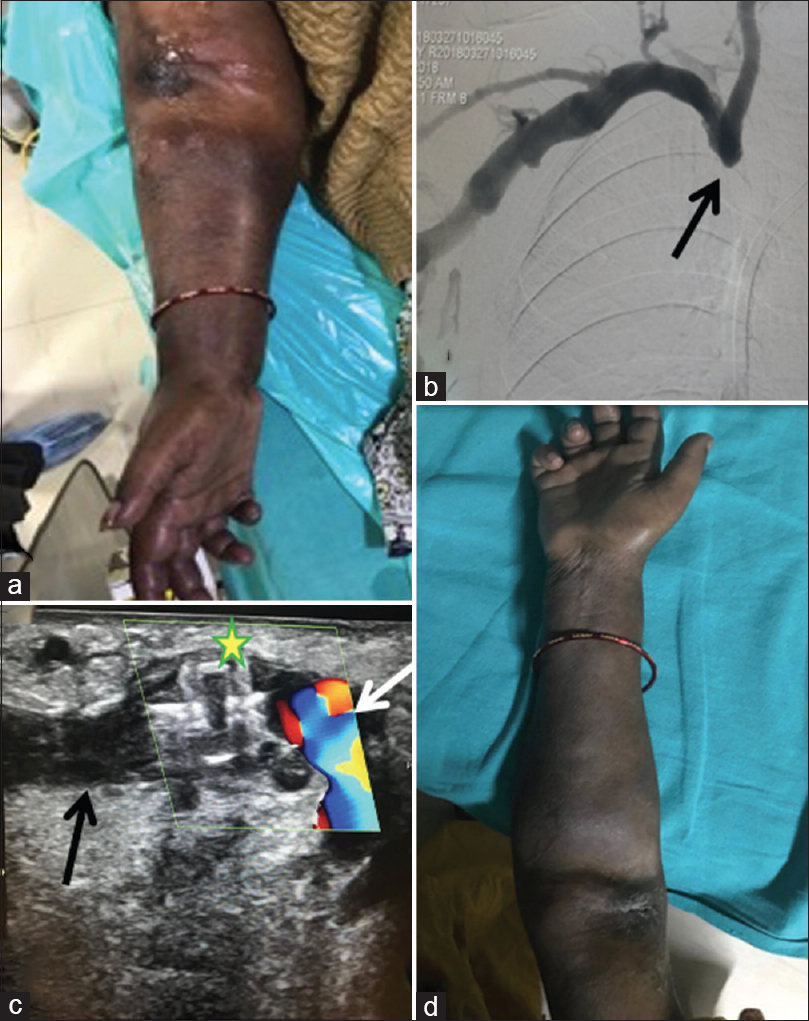
- 58-year-old female with right brachiocephalic AVF with intractable arm edema of the right arm (a), digital subtraction angiogram showing chronic occlusion of the right brachiocephalic vein (black arrow, b), USG image shows Amplatzer vascular plug (yellow star*) in situ with flow in brachial artery (white arrow) and no flow within the venous end (black arrow) of the AVF at the juxta-anastomotic site (c). A post procedure day 2 image of the right arm of patient showing resolution of the edema and clinical improvement (d)
Technical and clinical outcome
Two out of six patients who underwent embolization of reverse flowing basilic vein [Figure 3] in forearm had symptomatic relief from VH symptoms in 3 days. Remaining four patients of nonmaturing of AVF showed good flow across the fistula at the 1-month follow-up, thereby assisting in AVF maturation.

- (a-d): Accessory vein embolization. (a) Fistulogram shows retrograde filling of radial artery (arrow) with juxta-anastomotic stenosis. (b) Post balloon angioplasty check run shows that AVF flow but diverted into collateral veins via large accessory vein (arrowhead). (c and d) Accessory vein coiling (arrow, c) with restoration of flow in cephalic vein (white arrow, d). (e-h) Flow reduction procedure. (e) Left brachiocephalic vein occlusion, failed recanalization via jugular (white arrow) and femoral (black arrow) routes. (f) Fistulogram shows normal flow across left BCF. (g) Vascular plug deployed in outflow vein at the juxta-anastomotic segment (inset with star). (h) Post partial embolization fistulogram shows forward flow suggestive of patent AVF
Three patients who underwent embolization for flow reduction showed mean preprocedural flow volume of 650 ml/min and mean post procedural flow volume 360 ml/min post 24 h of procedure on Doppler ultrasonography. Post procedure, all these patients underwent successful dialysis (flow volume of 240–300 ml/min) with the same AVF on the second day and with the instruction of only minimal duration manual compression at needle insertion site post dialysis. At 6-month follow-up, significant improvement was seen in the form of resolution of symptoms of VH clinically in all these patients.
In patients of AVF closure, significant clinical improvement was seen immediately post-procedure followed by complete thrombosis of AVF within 24 h on USG in 17 out of 21 cases [Figure 4]. Four patients required an additional intervention in the form of thrombin injection and among them, one patient required a third setting of percutaneous NBCA glue injection, to achieve complete thrombosis of AVF.
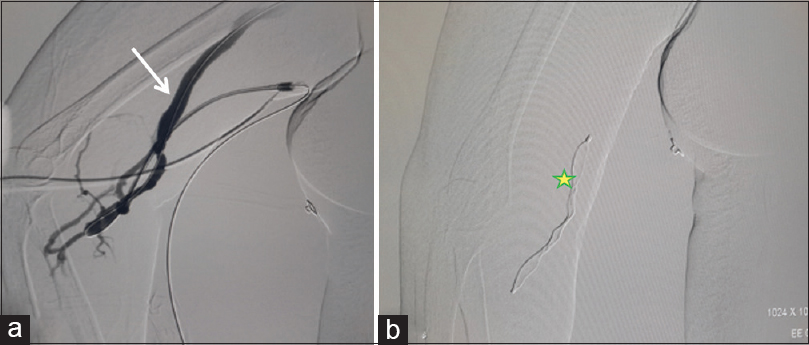
- 45-year-old female with right brachiocephalic fistula. Digital subtraction angiogram from the sheath placed at the juxta-anastomotic site shows filling of the cephalic vein (white arrow) (a), and deployment of the AVP plug (yellow star *) via sheath seen (b)
VP plug migration (proximally into cephalic vein) was seen in two patients [Table 1-Case no 1 and 12] likely due to under sizing; hence, they required additional secondary intervention in the form of percutaneous thrombin or NBCA injection. Percutaneous thrombin or NBCA injection was done by inflating a high-pressure noncompliant balloon (Conquest, Bard, rated burst pressure, 30 atm) endovascularly in the brachial artery across the AVF, prior to percutaneous injection of the embolic agent. Balloon inflation was done to prevent any reflux of embolic material into the artery and to avoid nontarget embolization.
| Case No | Age in years/Gender | Access type | Indication | Alternate dialysis method | VP diameter, mm | Plug type | Adjunct procedure | Access age, months | Follow-up | No of interventions |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56/M | Left BCF | CVO with ulceration With aneurysmal dilatation of vein |
Right BCF | 12 | II | Percutaneous Thrombin | 66 | 9, died | 4 |
| 2 | 58/F[Figure 2] | Right BCF | CVO with edema | TK | 12 | II | - | 16 | 12 | 2 |
| 3 | 54/F | Left BCF | CVO with edema | Right BCF | 10 | II | - | 34 | 16 | 3 |
| 4 | 65/F | Left BCF | CVO with ulceration | TK | 16 | II | - | 36 | 12, died | 4 |
| 5 | 66/M | Left BCF | CVO with ulceration | Right BCF | 12 | II | - | 42 | 10 | 3 |
| 6 | 54/M | Left BCF | CVO with ulceration and aneurysmal dilation of vein | TK | 10 | II | - | 28 | 13 | 2 |
| 7 | 45/F[Figure 4] | Right BCF | CVO with edema, DASS with ischemia, surgical closure failure | TK | 8 | II | Percutaneous Thrombin for reverse flowing collateral | 12 | 17 | 4 |
| 8 | 59/F | Left BCF | CVO with ulceration, surgical closure failure | Right BCF | 10 | II | - | 31 | 11 | 3 |
| 9 | 89/M | Left BCF | CVO with ulceration | CAPD | 12 | II | - | 38 | 6 | 2 |
| 10 | 78/F | Left BCF | CVO with edema | Permacath | 8 | IV | - | 22 | 8 | 2 |
| 11 | 54/F | Left BCF | CVO with edema | Right BCF | 12 | II | - | 36 | 10 | 2 |
| 12 | 28/F | Left BCF | CVO with ulceration | TK | 12 | II | 1st Balloon assisted Percutaneous Thrombin, 2nd glue | 60 | 10 | 3 |
| 13 | 50/M | Right BCF | CVO with edema | TK | 12 | II | - | 17 | 12 | 3 |
| 14 | 59/M | Left RCF | CVO with ulceration | Preemptive Transplant | 10 | II | - | 8 | 10 | 3 |
| 15 | 34/F | Right BCF | CVO with edema | TK | 11 | II | Percutaneous Thrombin | 28 | 8 | 2 |
| 16 | 56/F | Left BCF | CVO with edema | Right BCF | 12 | II | - | 16 | 11 | 3 |
| 17 | 65/M | Left BCF | CVO with edema | Permacath | 8 | II | - | 22 | 9 | 3 |
| 18 | 44/F | Left BCF | CVO with edema | Right BCF | 8 | IV | - | 24 | 6 | 2 |
| 19 | 47/F | Left BCF | CVO with edema | Right BCF | 8 | II | - | 28 | 9 | 3 |
| 20 | 56/M[Figure 1] | Left BCF | CVO with edema | Right BCF | 12 | CERA | - | 17 | 10 | 3 |
| 21 | 55/M | Left BCF | CVO with edema | Right BCF | 12 | II | - | 19 | 13 | 3 |
| 22 | 31/F | Left BCF | Flow reduction, aneurysmal dilatation of cephalic vein | Permacath | 10 | CERA | - | 16 | Prolonged compression caused thrombosis | 2 |
| 23 | 45/F | Left BCF | Flow reduction, aneurysmal dilatation of cephalic vein | - | 6 | CERA | - | 12 | 14 | 2 |
| 24 | 55/F[Figure 3e-h] | Left BCF | Flow reduction, CVO with edema | - | 8 | II | - | 14 | 12 | 2 |
| 25 | 51/M | Left BCF | Flow diversion, CVO with edema and reverse flowing basilic vein | - | 10 | IV | - | 18 | 6 | 3 |
| 26 | 58/M | Left BCF | Flow diversion, CVO with edema and reverse flowing basilic vein | - | 6 | IV | - | 36 | 6 | 2 |
| 27 | 44/F | Left BCF | Flow diversion, nonmaturing | - | coils | - | 14 | 6 | 2 | |
| 28 | 56/F | Left RCF | Flow diversion, nonmaturing | - | coils | - | 12 | 6 | 2 | |
| 29 | 44/M[Figure 3a-d] | Left RCF | Flow diversion, nonmaturing | - | coils | - | 15 | 6 | 2 | |
| 30 | 43/M | Left RCF | Flow diversion, nonmaturing | - | coils | - | 13 | 6 | 2 |
BCF-Brachiocephalic fistula, CVO-Central vein obstruction, VP-Vascular plug, TK-Transplant kidney
Except for VP migration, no obvious periprocedural complications were noted in any of the patients. There was no evidence of any distal ischemia, arterial thrombosis, perfusion deficits, or AVF recanalization (post VP closure) in any of the patients in the follow-up period.
All the patients with CVO and VH experienced a marked decrease in swelling and edema of the affected extremity within a week after occlusion of the fistula.
The mean follow-up time was 11 months, and follow-up ranged from 6 months to 17 months. Two patients with CVO [Table 1- Case no 1 and 4] died due to causes (chest infections) unrelated to fistula.
Discussion
Accessory veins are responsible for a minority of the primary AVF failures and are often associated with stenotic lesions. Nassar et al. and Beathard et al. in their case series have reported that a majority of the primary AVF failure cases with accessory draining veins could be salvaged by angioplasty of vascular stenosis and accessory vein obliteration.[1718] Our results also demonstrate similar findings; however, small sample size may be a limitation of our study.
Central venous stenosis leading to symptoms of VH in the ipsilateral extremity and chest wall are frequently encountered problems in patients on hemodialysis. Frequent use of temporary and tunneled catheters as a vascular access for long duration has been advocated as the most frequent cause of this CVO. These patients with CVO go on to develop severe edema, skin thickening, skin necrosis, and other changes related to VH. In our study, 17 out of 21 patients who presented with CVO had ipsilateral tunneled (n = 12) and nontunneled (n = 5) catheters. Preferred treatment for central vein stenosis is angioplasty with or without stenting. However, most of the chronic stenosis are difficult to treat. Revascularization of these stenoses by means of open surgery carries a very high risk of morbidity as ESRD patients are already associated with many comorbidities. Hence, after repeated failed attempts at central vein recanalization and in patients who are subsequently developing symptoms of VH, AVF closure with alternative method of dialysis is the option. Closure of the fistula was commonly performed using surgical techniques, through dissection of the area of the anastomosis and ligation of the fistula under local anesthesia, which involved longer hospital stays and raised morbidity. With rising awareness, minimally invasive techniques of endovascular closure have developed steadily.
Filippo et al. have mentioned successful closure of AVF in three patients of dialysis-associated steal syndrome with critical hand ischemia and intractable ipsilateral edema using Amplatzer vascular plug (AVP; St. Jude Medical, St. Paul, MN, USA).[9]
Gumus et al. have reported mid-term follow-up results of percutaneous embolization of AVF with AVP in 21 patients and concluded that AVP is a safe and effective device for the endovascular AVF closure.[19]
In the study published by Bui et al., six patients with problematic arteriovenous access underwent access occlusion using the AVP, and thus have concluded that AVP is minimally invasive and efficacious method for embolization of problematic AVFs.[20]
Sometimes very high flow AVFs can lead to symptoms of VH, hemodialysis access-induced distal ischemia, and high output heart failure and the radical treatment for these is access closure. At times, these AVF cannot be sacrificed as, with no alternative routes to dialysis, e.g., all central veins are stenosed or a solitary precious right BCF or poor candidate for renal transplant or central vein revascularization surgery. In these case scenarios of precious access, where the patency of AVF is desirable, flow reduction procedures are done and the flow volumes are calculated on Doppler pre and post procedure.[21] Various conventional methods of flow reduction include banding, fistula plication, and graft interposition. Bourquelot et al. in their study have described use of VP for flow reduction in problematic AVF. [2223]
A variety of embolizing agents are available, e.g., Coils and NBCA, detachable and occluding balloons; however, these agents show increased risk of migration and nontargeted embolization. In contrary, the VP is easy to deploy, less chances of migration if appropriately sized and associated with fewer complications. [122425]
The plug device has been widely used in the peripheral vascular interventions; however, its use in the closure of problematic hemodialysis fistulas has scarce reference in literature.
Owens et al. used an AVP for closure of problematic dialysis fistula in six patients, which included assisted coil embolization of collaterals in three patients, thus sparking the idea of assisted plug and coil embolization technique in the initial days.[16] Ozyer et al. shared their experience of AVP plug embolization in six patients of complicated dialysis fistulas with CVO in four out of six patients.[26] They mentioned that apart from the VP plug, coil and NBCA were also needed in two out of six patients to achieve complete stasis of flow. A study by Bui et al. has mentioned their initial experience in endovascular embolization of complicated hemodialysis access in six patients with AVP.[20] The main indication for the procedure in their study was DASS in five patients and an enlarging aneurysm in one patient. Powell et al. have mentioned their early experience in using AVP II in seven patients.[27] The main indications for access closure were DASS, high-flow tributaries, and limb swelling. The difficulties with surgical ligation in most of these studies were stated to be surgical failure, delayed wound healing, and complex anatomy. In this study, two patients of AVF closure were due to failure of surgical ligation and the likely reason was the severity of arm edema and multiple small collaterals in arm posing a technical challenge in identifying the culprit draining vein. The above-mentioned studies have broadly stated satisfactory results with the use of AVP in small groups of patients with short-term follow-up.
Thus, on comparing our results with the other studies we could say that VP is a safe and effective device for endovascular occlusion of these problematic AVFs. The VP contours to the shape of the adjacent vessel and can be resheathed and repositioned several times until the desired targeted location is achieved prior to final detachment. Our results have also shown that the VP is a good option for high flow AVFs where the risk of using coil and NBCA is likely to result in nontargeted embolization or distal migration.
Conclusion
Endovascular embolization for accessory vein obliteration, flow reduction, and fistula closure is an excellent alternative in selected patient population in comparison to surgical ligation given its minimally invasive nature. It is important to note that most of these ESRD patients show delayed wound healing; thus, a surgery requiring a wide incision would add to the morbidity of the patient. The sample size maybe a limitation in the study but the results are promising as far as the management of complicated hemodialysis fistulas is concerned.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- KDOQI vascular access workgroup. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48:S176-247.
- [Google Scholar]
- US renal data system 2015 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):Svii, S1-S305.
- [Google Scholar]
- Complications of arteriovenous haemodialysis access: Recognition and management. J Vasc Surg. 2008;48(5 Suppl):S55-80.
- [Google Scholar]
- ASDIN Clinical Practice Committee. Classification of complications associated with hemodialysis vascular access procedures. A position statement from the American society of diagnostic and interventional nephrology. J Vasc Access. 2008;9:12-9.
- [Google Scholar]
- High output cardiac failure due to brachiocephalic arteriovenous haemodialysis fistula: Two cases. Am Surg. 1998;64:239-41.
- [Google Scholar]
- Early arteriovenous fistula failure: A logical proposal for when and how to intervene. Clin J Am Soc Nephrol. 2006;1:332-9.
- [Google Scholar]
- Arteriovenous fistula failure: is there a role for accessory draining vein embolization? J Vasc Access. 2012;13:498-503.
- [Google Scholar]
- Management of venous hypertension following arteriovenous fistula creation for haemodialysis access. Indian J Urol. 2016;32:141-8.
- [Google Scholar]
- Hemodialysis arteriovenous access occlusion using the amplatzer vascular plug in patients with intractable arm edema. Case Rep Nephrol Dial. 2017;7:63-72.
- [Google Scholar]
- Vascular access flow reduction for arteriovenous fistula salvage in symptomatic patients with central venous occlusion. J Vasc Access. 2012;13:157-62.
- [Google Scholar]
- Surgical versus percutaneous care of arteriovenous access. Semin Vasc Surg. 2007;20:167-74.
- [Google Scholar]
- Transcatheter coil embolization for steal syndrome in patients with hemodialysis access. Acta Radiol. 2009;50:28-33.
- [Google Scholar]
- Indications for the use of the Amplatzer vascular plug in interventional radiology. Radiol Med. 2008;113:707-18.
- [Google Scholar]
- Transcatheter occlusion of pulmonary arteriovenous malformations using the amplatzer vascular plug II. Cath Cardiovasc Interv. 2008;71:940-3.
- [Google Scholar]
- Dual use of an amplatzer device in the transcatheter embolisation of a large high-flow renal arteriovenous fistula. J Vasc Interv Radiol. 2007;18:671-6.
- [Google Scholar]
- Use of the amplatzer vascular plug as a coil constrainer during endovascular occlusion of a dialysis shunt. Cardiovasc Interv Radiol. 2007;30:754-6.
- [Google Scholar]
- Endovascular treatment of the “failing to mature” arteriovenous fistula. Clin J Am Nephrol. 2006;1:275-80.
- [Google Scholar]
- Percutaneous embolization of hemodialysis fistulas by AMPLATZER vascular plug with midterm follow-up. J Vasc Interv Radiol. 2011;22:1581-5.
- [Google Scholar]
- Amplatzer vascular plug for arteriovenous hemodialysis access occlusion: Initial experience. J Vasc Access. 2009;10:5-10.
- [Google Scholar]
- Accuracy of volumetric flow rate measurements: An in vitro study using modern ultrasound scanners. J Ultrasound Med. 2009;28:1511-8.
- [Google Scholar]
- Graft inclusion technique: A new flow reduction procedure for high flow arteriovenous fistulae. Ann Vasc Dis. 2018;11:202-9.
- [Google Scholar]
- Amplatzer vascular plug for occlusion or flow reduction of hemodialysis arteriovenous access. J Vasc Surg. 2014;59:260-3.
- [Google Scholar]
- Treatment of steal syndrome in a distal radio cephalic fistula using intravascular coil embolisation. J Vasc Surg. 2008;47:457-9.
- [Google Scholar]
- Flow interruption of the distal radial artery: Treatment for finger ischemia in a matured radio cephalic AVF. J Vasc Access. 2008;9:58-63.
- [Google Scholar]
- Application of the amplatzer vascular plug in endovascular occlusion of dialysis accesses. Cardiovasc Intervent Radiol. 2009;32:967-73.
- [Google Scholar]
- Early experience with the amplatzer vascular plug II for occlusive purposes in arteriovenous haemodialysis access. Cardiovasc Intervent Radiol. 2010;33:150-6.
- [Google Scholar]







