Translate this page into:
Existing and Evolving Therapies for Arteriovenous Fistula and Graft Dysfunction
Corresponding author: Priti Meena, Department of Nephrology, All India Institute of Medical Sciences (AIIMS) Bhubaneswar, India. E-mail: pritimn@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vachharajani TJ, Bhargava V, Sequeira A, Meena P. Existing and Evolving Therapies for Arteriovenous Fistula and Graft Dysfunction. Indian J Nephrol. 2024;34:552-60. doi: 10.25259/ijn_528_23
Abstract
A functional vascular access (VA) is of paramount importance to patients on hemodialysis therapy. While arteriovenous fistula (AVF) remains the preferred VA, their long-term patency is unpredictable. A dysfunctional VA contributes to a high morbidity rate, an increased susceptibility to major adverse cardiovascular events, recurrent hospitalization, and a poor quality of life. The recent innovations in devices and technologies have significantly expanded our options to create and prolong VA patency. Endovascular devices such as WavelinQ and Ellipsys are recent additions to creating a VA. The endovascular creation of AVF helps reduce the wait time and potentially avoids or reduces the duration of catheter use. The bioengineered graft and immediate access arteriovenous graft offer reasonable alternatives in a select group of patients. There is growing evidence that covered stents and drug-coated balloons offer options to prolong the VA patency. Finally, the role of stem cell therapy in VA is currently being explored. This article presents a comprehensive review of the conventional and current developments in the management of a dysfunctional VA.
Keywords
Vascular access
End-stage kidney disease
Hemodialysis
Endovascular AVF
Dysfunctional vascular access
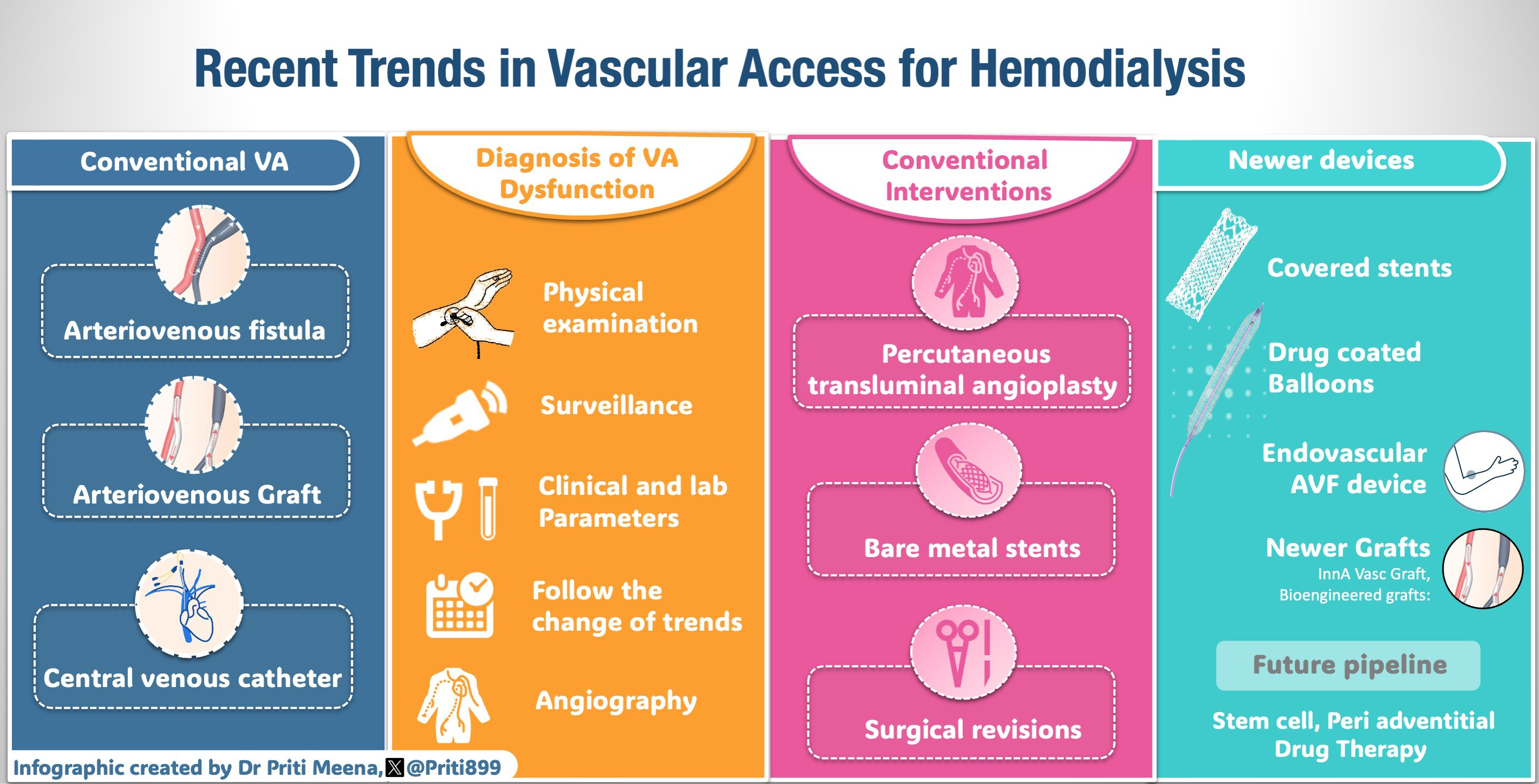
Introduction
The importance of well-functioning vascular access (VA) was brought to the forefront in the early 2006 when the first Clinical Practice Guidelines were published by the National Kidney Foundation – Kidney Disease Outcomes Quality Initiative (NKF-KDOQI group).1 The revised 2020 guidelines emphasize a patient-focused approach and recommend formulating an end-stage kidney disease (ESKD) life plan.2 The transition from “fistula first” approach to “right access in the right patient” evolved from the experiences gathered over the last two decades. The ESKD life plan highlights the importance of aligning the VA plan with the patient’s preferences, needs, and overall health condition. An arteriovenous fistula (AVF) is still the preferred primary VA for most due to its relatively longer patency, and low risk for infection and thrombosis compared to arteriovenous (AV) graft but at a cost of a higher primary failure rate.3
VA dysfunction stands as a prominent contributor to morbidity in dialysis in individuals with ESKD.4 Kuo et al. showed that the presence of VA dysfunction was associated with a 1.385-fold increase in the likelihood of experiencing major adverse cardiovascular events (MACE) in hemodialysis patients.5 Additionally, recurrent dysfunction in VA may serve as a predictive indicator for composite all-cause mortality.6 The economic impact of VA failure is substantial, contributing significantly to healthcare costs, primarily due to the substantial proportion of hospitalizations within the hemodialysis population.7 Thus, a dysfunctional VA remains a burden to the patient and society.8
The current article provides an in-depth review of the pathogenesis of VA failure, conventional and recent developments in the field of VA. The article discusses techniques to diagnose a dysfunctional VA and offers insight into the innovative devices that are available.
Pathophysiology of VA failure
Primary failure is defined as AVF that fails to develop adequately to support dialysis or fails within the first three months of its use. This occurs in 30–50% of cases.9 Neointimal hyperplasia (NIH) causing stenosis is the main culprit. Roy-Chaudhury et al. highlighted the role of upstream and downstream events in NIH development.10 The upstream factors include trauma during fistula creation such as vessel manipulation during surgery, hemodynamic stress at the artery-vein anastomosis due to turbulence and low shear stress, arterial injury, vessel compliance mismatch, preexisting comorbid conditions, systemic inflammation, and abnormal flow conditions after anastomosis. Downstream factors are mostly biological events secondary to upstream factors causing endothelial dysfunction, smooth muscle cell injury, and poor vascular remodeling. ESKD is itself a predisposing factor for thrombosis. In patients with ESKD, hypercoagulability is attributed to endothelial dysfunction induced by cytokines and systemic inflammatory stress.11 Repeated VA puncture can cause platelet thrombi formation and cytokine release. In addition, poor technique of canulation, such as inappropriate needle size, the wrong angle of needle insertion, and the wrong direction of the needle bevel, also adversely affects AVF and graft survival.12
The current KDOQI guidelines does not provide a minimum threshold diameter of artery and vein to create an AVF, but suggest that vessel diameter of 2 mm or less be carefully evaluated for feasibility and quality before performing AVF surgery. In addition, it is also reasonable to examine characteristics of vessel quality such as distensibility.2
The presence of an indwelling central vein catheter (CVC) or pacemaker ipsilateral to the VA predisposes to an increased risk of central venous stenosis and reduced VA patency.13 The common location of the stenosis encountered depends on the access location. Radio-cephalic (RC) AVFs tend to be more susceptible to inflow stenosis, whereas brachio-cephalic (BC) AVFs are more prone to outflow stenosis,14 such as cephalic arch stenosis (CAS). The arch moves through the dense clavipectoral fascia and has limited capacity to dilate, resulting in the narrowing of the lumen. Other factors, such as turbulence and shear stress related to the curve, also contribute to intimal injury at the cephalic arch.15 The common site for NIH in arteriovenous grafts (AVG) is at the vein graft anastomosis.10
Diagnostic tools
Early diagnosis of a dysfunctional AV access can help with timely intervention to: (i) prolong the AV access patency; (ii) avoid disastrous complications such as thrombosis; (iii) prevent or minimize CVC use; (iv) potentially avoid hospitalization, and (v) reduce healthcare costs. Several complementary tools utilized to diagnose a dysfunctional AV access include:
Physical examination or monitoring technique
Surveillance technique
Trending clinical parameters
An important practical point common to all of these tests is the need to observe trends. A change over time is more specific than a single snapshot evaluation.
Monitoring technique
The physical examination of an AV access is a simple bedside tool that is practical, quick, reliable, low cost, and allows engaging the entire dialysis community, including the patient. The physical examination follows the principles of inspection, palpation, and auscultation (look, listen, and feel) and takes about a minute to perform.16,17 The skin over the AV access is examined for discoloration, redness, rash, infected scabs, purulent discharge, formation of an aneurysm or pseudoaneurysm, bruises, and infiltration or swelling as signs of infection or obstruction.18 A normal AV access feels soft along the outflow vein with a continuous thrill. The thrill is most prominent at the anastomosis and weakens as one moves the palpating finger proximally toward the heart. The presence of a loud bruit with systolic accentuation or its absence in diastole suggests an underlying stenosis.
Additional tests performed frequently are the arm elevation test to see a collapsing outflow vein, suggestive of a wide-open outflow track. Any stenosis in the outflow track leads to engorgement and failure of the vein to collapse with arm elevation. Patients can participate in their own care by monitoring their AV access with a simple arm elevation test at home.
An augmentation test is performed to assess the adequacy of the inflow segment of the access. Any change in the physical examination findings needs to be monitored closely before the AV access develops thrombosis.
Surveillance technique
Surveillance is defined as measuring either the blood flow or pressure within an AV access. Frequently used techniques to measure VA blood flow are ultrasound dilution and conductivity dialysance. Studies on the technique of blood flow measurement technique have remained controversial and inconclusive, especially when the endpoint is predicting access thrombosis or long-term patency. In clinical practice, trending monthly access blood flow is a reasonable complement to changes in physical examination.19,20
Access pressure measurements can be dynamic or static. Dynamic pressure measurements are again very controversial.19,21 Automated trending of normalized access pressure to mean arterial pressure measurements have limited benefit in predicting a thrombotic event. The measurement of static VA pressure is more predictable, but the technique to measure is cumbersome and impractical.
The use of Doppler ultrasound (DUS) for surveillance is an excellent tool if one can perform frequent evaluations in the dialysis center. The DUS is excellent for measuring VA blood flows and identifying anatomical lesions in the peripheral veins, but not ideal for central vein lesions. Additionally, it requires the dialysis staff to be trained.22
If available, surveillance tools are complementary to monitoring techniques. The key takeaway message is to trend the results and intervene per KDOQI guidelines: Drop in access blood flow by 25% from baseline. Reduction in access blood flow below 400 ml/min in an AVF or 600 ml/min in AVG, along with clinical signs of hemodynamic changes.2
Clinical Parameters
Trending clinical parameters on a regular basis should be part of the algorithm to detect dysfunctional AV access. Monitoring monthly solute clearance provides clues toward possible underlying dysfunctional AV access. Other frequently encountered reasons that would suggest dysfunctional AV access are (1) repeated difficulty with needle cannulation and (2) prolonged bleeding, defined as > 15 min post-needle withdrawal, is suggestive of increased venous hypertension from a proximal stenosis. Similarly, high arterial or venous pressures triggering machine alarms may be suggestive of stenosis.23
Existing treatment options
Angiogram and angioplasty
For a long time, balloon angioplasty (PTA) has been the gold standard, even though it does not alter the biology of the lesion. The presence of hemodynamic changes and an anatomic narrowing of > 50% of the vessel lumen is commonly treated with PTA. During PTA, while the vessel is mechanically dilated, it disrupts the inner architecture of the vessel. In the long run, this results in increased vessel stiffness, increased cellular proliferation activity, and recurrence of the lesion requiring more interventions.24,25 While the immediate success rates for PTA in AVFs versus AVGs range from 89.5% to 97% and from 80% to 98%, respectively, the results are not sustained, being in favor of AVFs.26 The one-year post angioplasty primary patency (defined as the period from the initial angioplasty until a second angioplasty is performed) for AVFs ranges from 40% to 60% and from 31% to 45% for AVGs.26,27 The challenge with angioplasty is that the lesion frequently tends to recur and may require repeated endovascular interventions. The benefit of angioplasty is that the technique is simple, can result in immediate improvement of access blood flow, and allows the patient to continue dialysis therapy on the same day.
Conventional PTA has limitations, such as the inability to treat resistant and elastic lesions. High pressure (> 20 ATMs), ultra high pressure (> 30 ATMs), and cutting balloons (CBs) are used to treat the former.28 CBs act by exposing blades causing focal microsurgical intimal disruption of elastic and fibrotic tissue at low pressures.29 This causes less barotrauma, less NIH and less restenosis. In a meta-analysis of randomized control trials (RCTs) involving 1,034 patients comparing CBs to conventional angioplasty, the six-month target lesion patency was higher with CB angioplasty (67% versus 55%, p < 0.05).28 Similar results were noted when using these balloons at the VGA of grafts (86% versus 56%).30 Beyond one year, the patency drops.30 Aneurysm formation, vessel dissection, and extravasation are known complications. CBs are also expensive.28
Stents
Covered stents are FDA approved for VA stenosis and recommended for use in venous rupture and recurrent, elastic lesions (defined as recurrent narrowing > 30% after full effacement with angioplasty).31 The latter recommendation is based on the observation that access survival is inversely related to the magnitude of residual stenosis post angioplasty.32 FLAIR pivotal33 and RENOVA34 trials showed covered stents provided better target lesion primary patency (TLPP) as well as greater freedom from reinterventions over conventional angioplasty in non-thrombosed AVGs. The recent AVeVA study (using the Covera stent) also demonstrated better TLPP and fewer target lesion reinterventions in AVGs.35 Unlike the previous two studies, it enrolled patients with both thrombosed and non-thrombosed AVGs; in this sense, it is like the REVISE trial that utilized the Viabahn covered stent.36 The latest AVeNEW study used the same Covera stent in stenosed AVFs with similar better TLPP for over 24 months compared to the PTA group.37 Further, longer mean time between interventions at the target lesion as well as fewer target lesion interventions were needed in the stent group at 24 months. RCTs, though limited, have shown better TLPP with stent grafts (SGs) compared to PTA at the cephalic arch.38
The stainless steel bare metal stents (BMS) in the 1990s did not appear to show any superiority over PTA in RCT of the cephalic arch.39 Most studies also do not suggest they are beneficial in central vein stenosis either.40 They are prone to in-stent restenosis and require multiple reinterventions.41,42 Subsequent nitinol BMS with its shape memory and flexibility showed improved patency rates over PTA.43,44 Even though RCTs comparing BMS and SGs are few, SGs showed better patency.45
Stents may migrate, fracture, have their struts protrude through the skin, and cause extrinsic compression of the adjacent artery.46,47 In the two studies by Haskal et al., the risk of distal hypoperfusion ischemic syndrome increased in the stent group.33,34 Infection is another complication, especially when the stent is placed within a graft and across a pseudoaneurysm.48 Lastly, their high cost is offset by fewer reinterventions, especially with thrombosed AVGs.49
Drug-coated balloons
The latest technology employed to tackle NIH is the use of drug-coated balloons (DCB). The balloon is coated with either sirolimus or paclitaxel, an antiproliferative agent, which minimizes inflammation and prevents migration of vascular smooth muscle cells and fibroblasts into the intima.50 This reduces restenosis and provides longer patency. The RCT by Lookstein et al. compared a paclitaxel DCB with conventional PTA in 330 patients with fistulas.51 Seventy percent of them had re-stenotic lesions. The six-month TLPP and access circuit primary patency (ACPP) were better in the DCB group. In addition, the number of procedures to maintain target lesion and access circuit patency was also reduced. A similar trend was noted at 24 months with DCB delaying repeat angioplasty in 50% of patients by 15 months (presented at Charing Cross Meeting, 2021). DCBs have also shown benefits by decreasing reintervention episodes in central vein stenosis and in-stent restenosis from nitinol BMS.52,53 The dose of paclitaxel, the excipient used, and the inflation times appear to matter. Lower drug dosage and inflation times as well as nonurea excipient may not provide benefit.54,55 DCBs do not benefit stenoses that are not NIH-driven. They are used in areas where stenting is not an option and unlike a stent, they do not leave behind a permanent implant. They are expensive as well.50 Figure 1 shows the schematic diagram of drug-eluting balloon angioplasty.
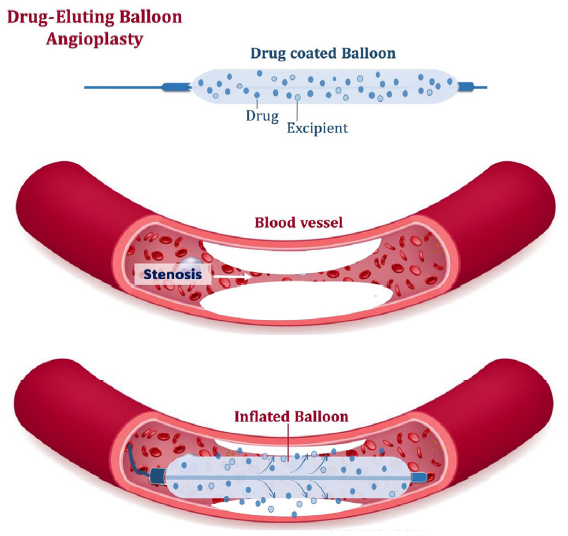
- The schematic diagram of drug-eluting balloon angioplasty.
Pharmacological interventions
Pharmacological interventions have been studied to maintain access patency. In these studies, the dosage and duration are not standardized. Further, the results differ between AVFs and AVGs. Aspirin (ASA) prevents platelet aggregation while fish oil reduces blood viscosity, promotes vasodilation, inhibits smooth muscle cell proliferation, and reduces inflammation.56 The FAVOURED trial, an RCT of 560 patients, use of either agent (8 gm fish oil, 100 mg ASA) failed to prevent primary AVF failure.56 The use of 4 gm of fish oil in the FISH study reduced the thrombotic events by half as well as the number of interventions to maintain patency in newly placed AVGs.57 The use of antiplatelet agents like clopidogrel prevents early fistula thrombosis without increasing the proportion of fistulae suitable for dialysis, suggesting that just maintaining patency is not enough for maturation.58 In newly placed AVGs, the use of dipyridamole and ASA (DAC study) prolonged primary patency and reduced the rate of stenosis without improving cumulative patency.59 Gastrointestinal (GI) and cannulation site bleeding are recognized complications with dual antiplatelet agents.60 Hence, these are to be avoided in patients at high risk for bleeding. P2Y12 receptor blockers are more effective in maintaining primary AVG patency than ASA.61 Dual antiplatelets are reserved for patients with a high risk for early graft thrombosis.2 The use of low-dose warfarin also increases bleeding risk without improving the patency of AVGs.62
Evolving therapies
Bioengineered grafts
Grafts created from patients’ own tissues (autologous graft) may have a better potency rate than synthetic grafts. Canine aortic transplant experiments dating back to 1948, were one of the first experimental models attempting bioengineered vascular tissue.18 Studies have evaluated the feasibility of using in vitro bioengineered hemodialysis grafts. A multicenter study enrolled ten patients with ESKD to receive completely autologous tissue-engineered grafts for hemodialysis.19 Most of the grafts were able to be used for dialysis and the primary patency was 60% at six months. Three of these grafts failed in the first three months. Human acellular vessels cultured using human vascular smooth muscle cells in vitro on a biodegradable scaffold were placed in 60 patients with a mean follow-up period of 16 months. The patency was limited to a one-year primary patency, primary assisted patency, and secondary patency rates of 28%, 38%, and 89%.20
InnA Vasc Graft (IAVG)
The IAVG combines a standard expanded polytetrafluoroethylene (ePTFE) vascular graft with a novel graft modification technology engineered with materials that provide durable, self-sealing cannulation chambers with puncture-resistant posterior and sidewall surfaces. The penetration-resistant cannulation chambers are externally molded and bonded circumferentially to one contiguous segment of ePTFE and provide protection against expansion, deformation, and injury to the posterior and sidewalls of the graft.63 A dense, lightweight polymer extends along the length of the chamber on the posterior aspect to function as a needle-stopping back plate. The raised oval on the top of each chamber provides an easily identifiable cannulation zone, which when punctured in this area, ensures safe needle access to the graft lumen. This graft addresses many of the inadequacies in hemodialysis access. It has specific access chambers meant to prevent back or sidewall access. These cannulation zones have self-sealing technology to enable immediate use and eliminate the need for tissue incorporation. Data is sparse regarding the durability, thrombosis rates, and infection risks. Initial case reports indicated that the graft appeared to be functioning as intended.64 This feature is novel to hemodialysis (HD) access and has the potential to reduce acute needle-related injury due to technical error during cannulation and a feature that hypothetically could encourage patients and family members to consider home HD.
Hemodialysis reliable outflow graft
The Hemodialysis Reliable Outflow (HeRO) graft (Merit Medical, South Jordan, UT) was designed to address failing AVFs and AVGs in patients with central venous obstruction. The device comprises a 6 mm inner diameter ePTFE arterial graft component and a 5 mm inner diameter nitinol reinforced silicone venous outflow component.65 The venous outflow component is placed into the internal jugular vein and advanced until the radiopaque marker is in the mid- to upper-right atrium. The arterial graft is then tunneled from the brachial artery just proximal to the antecubital fossa, over the biceps muscle, to the deltopectoral groove. The graft is then attached to the outflow component using a titanium connector in an RCT, comparing the HeRO graft with conventional AV grafts in patients undergoing HD. The HeRO Graft has comparable rates of patency, dialysis adequacy, and bacteremia after 12 months.66 A systematic review on the outcomes of HeRO graft in HD patients showed that the primary and secondary pooled patency rates in this group were 21.9% (9.6–37.2%) and 59.4% (39.4–78%), respectively. The incidence of dialysis access-associated steal syndrome was rather low, at 6.3% (with a range of 1–14.7%). The occurrence of HeRO-related bacteraemia ranged from 0.13 to 0.7 occurrences per 1,000 days.67
Endovascular AVF creation
Endovascular AVF are minimally invasive and are associated with lesser damage to the endothelium. There are two available devices for endoAVF creation: WavelinQ 4 F (Beckton, Dickinson and Company, NJ, USA) and the Ellipsys Vascular Access System (Medtronic, San Juan Capistrano, CA, USA).68
EndoAVF device uses two catheters to create the AVF. One catheter is advanced into the brachial vein and the second into the brachial artery. On alignment in the radial artery and vein or ulnar artery and vein, the magnets in each of the vessels attract and hold each other together. Radiofrequency energy from the venous catheter creates a connection between the two vessels. The brachial vein should be embolized to ensure better maturation. The Ellipsys system creates an anastomosis using thermal energy and pressure by inserting the catheter through the perforator veins.69 The technical success rates are high (> 98%).68,70 The procedure time may be as short as 15 minutes. A functional AVF supporting HD was achieved > 95% of the time.71 The reported one-year primary, primary assisted, and secondary patency rates with the Ellipsys system are 54%, 85%, and 96%, respectively.69 The cumulative patency with the WavelinQ system has been shown to be 92.8% after one year and 91.6% at two years.71 In terms of the economics, the cost of managing complications such as stenosis, aneurysm, and infections is lower with endoAVF as compared to surgically created AVF.72–74 As per patient satisfaction analysis, endoAVF scores are higher than surgical AVF.71 Figure 2 shows WavelinQ device used for the creation of a radial artery to lateral radial vein fistula in the left forearm. Figure 3 shows Ellipsys device used for the creation of a radial fistula.
- WavelinQ device used for the creation of a radial artery to the lateral radial vein fistula in the left forearm. The arterial electrode is at the bottom. The venous catheter, above the arterial electrode, contains the electrode that delivers a burst of radio frequency energy to create the AVF in the target zone. AVF: Arteriovenous fistula. Courtesy: Dr Alejandro Alvarez.
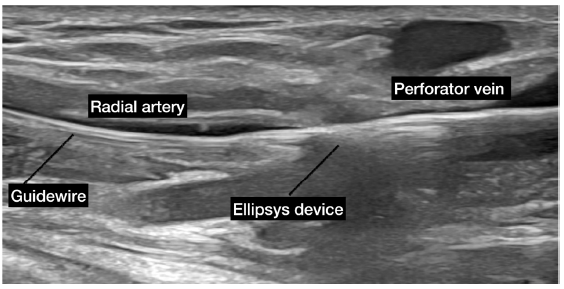
- Ellipsys device used for the creation of a radial fistula. The ellipsys device passes from the perforator vein to the radial artery along a guide wire. The two ends of the Ellipsys catheter are approximated to deliver a burst of thermal energy to create the AVF in the target zone. AVF: Arteriovenous fistula. Courtesy: Dr Naghae Mawla.
Stem cell therapy
Stem cells may have a role in one of the most important complications such as venous stenosis by reducing venous NIH.75 However, data on this application to AVF is very limited at present. Adipose-derived mesenchymal stem cells transplanted to the outflow vein after AVF creation reduce proinflammatory gene expression in an immunocompetent mouse model.76 This thereby could have a role in reducing venous stenosis. A clinical trial in 74 patients with AVFs is underway to test the safety and efficacy of autologous stem cell transplantation for preventing AVF failure.77 Mouse stem cells harvested from inbred C57BL6/J mice when delivered to adventitia of stenotic outflow vein after angioplasty has shown to reduce proinflammatory genes, reduce macrophages, smooth muscle cells, and fibroblasts in the vessel wall, thereby improving the latency of fistula.76
Peri-adventitial drug therapy
The vasa vasorum in the adventitia plays an important role in inflammatory cell migration to the intima, leading, in turn, to NIH.78 Direct delivery of the drug to the adventitia allows for a higher volume of drug delivery as compared to drug-eluting stents, since the drug delivered is washed due to the flow. Direct delivery to the intima may thus reduce neoinitimal hyperplasia by disrupting the mechanisms involved in the formation of stenosis in the vein.79,80 A 1-alpha, 25 (OH) D3 has been shown to reduce inflammation by playing a crucial role in cellular differentiation and immune responsiveness. This has been substantiated with animal models, such as in a pig model with CKD, where poly-lactic-co-glycolic acid nanoparticles encapsulated with 1,25 (OH) 2 D3 impregnated into the adventitia of outflow vein after creating an AV fistula showed reduced inflammation and apoptosis.81 Human studies are yet to elucidate the clinical efficacy of the modality.
Evolving techniques to aid AVF maturation
A significant number of AVF procedures fail due to the presence of small veins that have limited capacity for blood flow and distensibility. The utilization of an intermittent pneumatic compression device in the early stages may potentially facilitate the process of AVF dilatation.82 Noninvasive medical devices such as Fist Assist may have the potential to offer clinical benefits by contributing to fistula maturation.83 Intermittent pneumatic compression has demonstrated efficacy in many trials for promoting efficient dilatation of superficial veins, particularly in the forearm and upper arm cephalic veins.82,84 The application of the external support device VasQ during AVF creation has shown encouraging results in terms of patency and functionality.85 The device’s mechanical and geometrical characteristics provide a streamlined transition for a more laminar flow profile. These features reduce the possibility of juxta-anastomotic stenoses. The device is intended to produce the most uniform flow profile by providing ideal configuration elements, such as angulation and tapering.86 Additionally, it reinforces and protects the perianastomotic vein from elevated pressure, wall tension, and flow levels. Research conducted on rodents has revealed the significant regulatory function of nitric oxide (NO) in the development of AVF.87 Somarathna et al. found that the NO-releasing nano matrix gel reduced intimal hyperplasia by over 70%, increased vein diameter, and improved hemodynamic adaptation.88
This article highlights the pivotal role of VA dysfunction in the health outcomes of HD patients. It underscores the alarming impact of VA dysfunction on morbidity and mortality rates. Recent years have witnessed a transformative wave of advancements in the realm of VA care for HD, with innovative devices and technologies reshaping the treatment landscape. Emerging technologies like DCB, novel endovascular devices, and bioengineered grafts offer promising solutions to address the challenges posed by VA dysfunction. Additionally, the integration of stem cell therapy and the proactive involvement of dialysis community in early access monitoring further enhance the potential for improved patient care and outcomes. These developments pave the way for a brighter future in the management of VA dysfunction in HD patients, with a focus on optimizing VA and enhancing overall well-being.
Conflicts of interest
There are no conflicts of interest.
References
- Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48:S176-247.
- [CrossRef] [PubMed] [Google Scholar]
- KDOQI clinical practice guideline for vascular access: 2019 Update. Am J Kidney Dis. 2020;75:S1-164.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of outcomes with arteriovenous fistula and arteriovenous graft for vascular access in hemodialysis: A prospective cohort study. Am J Nephrol. 2016;43:120-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association of early failure of arteriovenous fistula with mortality in hemodialysis patients. Sci Rep. 2021;11:5699.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Association between vascular access dysfunction and subsequent major adverse cardiovascular events in patients on hemodialysis: a population-based nested case–control study. Medicine. 2015;94:e1032.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recurrent vascular access dysfunction as a novel marker of cardiovascular outcome and mortality in hemodialysis patients. Am J Nephrol. 2016;44:71-80.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7:523-35.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of haemodialysis outcomes. Nat Rev Nephrol. 2022;18:378-95.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487-94.
- [CrossRef] [PubMed] [Google Scholar]
- Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant. 2009;24:2786-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: A 2020 update. JCM. 2020;9:2359.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cannulation technique influences arteriovenous fistula and graft survival. Kidney Int. 2014;86:790-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Maturation of arteriovenous fistulas in patients with and without preexisting hemodialysis catheters. Ann Med Surg. 2019;48:11-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Inflow stenosis in arteriovenous fistulas and grafts: a multicenter, prospective study. Kidney Int. 2005;67:1986-92.
- [CrossRef] [PubMed] [Google Scholar]
- Cephalic arch stenosis in autologous hemodialysis fistula; to stent or not to stent? Long-term follow up. Egypt J Radiol Nucl Med. 2022;53:96.
- [Google Scholar]
- Physical examination of arteriovenous fistula. Last Accessed Aon August, 31 2023. Available at https://www.youtube.com/watch?v=m1-C61AOY3Q
- [Google Scholar]
- Diagnosis of arteriovenous fistula dysfunction: Diagnosis of AVF dysfunction. Semin Dial. 2012;25:445-50.
- [CrossRef] [PubMed] [Google Scholar]
- Atlas helps renal staff identify access problems. Nephrol News Issues. 2011;25:24.
- [PubMed] [Google Scholar]
- A multicenter randomized clinical trial of hemodialysis access blood flow surveillance compared to standard of care: the hemodialysis access surveillance evaluation (HASE) study. Kidney Int Rep. 2020;5:1937-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The hemodialysis access surveillance controversy continues. Kidney Int Rep. 2020;5:1848-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A meta-analysis of randomized clinical trials assessing hemodialysis access thrombosis based on access flow monitoring: Where Do We Stand? Seminars in Dialysis. 2015. ;28 [cited February 5, 2024]. Available from: https://onlinelibrary.wiley.com/doi/10.1111/sdi.12342
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of doppler ultrasonography in the evaluation of hemodialysis arteriovenous access maturation and influencing factors. J Vasc Access. 2021;22:42-55.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- How is Arteriovenous Fistula Longevity Best Prolonged?: The Role of Surveillance. Asif A, editor. Semin Dial. 2015;28:33-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Angioplasty induced changes in dialysis vascular access compliance. Ann Biomed Eng. 2021;49:2635-45.
- [CrossRef] [PubMed] [Google Scholar]
- Highly increased cell proliferation activity in the restenotic hemodialysis vascular access after percutaneous transluminal angioplasty: implication in prevention of restenosis. Am J Kidney Dis. 2004;43:74-84.
- [CrossRef] [PubMed] [Google Scholar]
- Choosing the right treatment for the right lesion, part I: A narrative review of the role of plain balloon angioplasty in dialysis access maintenance. Cardiovasc Diagn Ther. 2023;13:212-32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Quality improvement guidelines for percutaneous image-guided management of the thrombosed or dysfunctional dialysis circuit. J Vasc Interv Radiol. 2016;27:1518-30.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of cutting balloon angioplasty and percutaneous balloon angioplasty of arteriovenous fistula stenosis: A meta-analysis and systematic review of randomized clinical trials. J Interven Cardiology. 2015;28:288-95.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of cutting balloon angioplasty and percutaneous balloon angioplasty of arteriovenous fistula stenosis: A meta-analysis and systematic review of randomized clinical trials. J Interven Cardiology. 2016;29:334-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective, randomized study of cutting balloon angioplasty versus conventional balloon angioplasty for the treatment of hemodialysis access stenoses. J Vasc Surg. 2014;60:735-40.
- [CrossRef] [PubMed] [Google Scholar]
- The role of stents in hemodialysis vascular access. J Vasc Access. 2023;24:107-16.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Predictors of arteriovenous graft patency after radiologic intervention in hemodialysis patients. Am J Kidney Dis. 2001;37:945-53.
- [CrossRef] [PubMed] [Google Scholar]
- Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med. 2010;362:494-503.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective, randomized, concurrently-controlled study of a stent graft versus balloon angioplasty for treatment of arteriovenous access graft stenosis: 2-year results of the RENOVA Study. J Vasc Interv Radiol. 2016;27:1105-1114.e3.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective, multicenter clinical study of the covera vascular covered stent in the treatment of stenosis at the graft-vein anastomosis of dysfunctional hemodialysis access grafts. J Vasc Interv Radiol. 2022;33:479-488.e3.
- [CrossRef] [PubMed] [Google Scholar]
- Balloon angioplasty versus Viabahn stent graft for treatment of failing or thrombosed prosthetic hemodialysis grafts. J Vasc Surg. 2016;64:1400-1410.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective, randomized, multicenter clinical study comparing a self-expanding covered stent to percutaneous transluminal angioplasty for treatment of upper extremity hemodialysis arteriovenous fistula stenosis. Kidney Int. 2023;104:189-200.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized prospective study comparing outcomes of angioplasty versus viabahn stent-graft placement for cephalic arch stenosis in dysfunctional hemodialysis accesses. J Vasc Interv Radiol. 2015;26:1355-61.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective randomized trial of a metallic intravascular stent in hemodialysis graft maintenance. J Vasc Interv Radiol. 1997;8:965-73.
- [CrossRef] [PubMed] [Google Scholar]
- Editor’s choice – vascular access: 2018 clinical practice guidelines of the european society for vascular surgery (ESVS) Eur J Vasc Endovasc Surg. 2018;55:757-818.
- [CrossRef] [PubMed] [Google Scholar]
- Upper extremity central venous obstruction in hemodialysis patients: treatment with Wallstents. Radiology.. 1997;204:343-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of hemodialysis-related central venous stenosis or occlusion: Results of primary wallstent placement and follow-up in 50 patients. Radiology. 1999;212:175-80.
- [CrossRef] [PubMed] [Google Scholar]
- Balloon angioplasty vs nitinol stent placement in the treatment of venous anastomotic stenoses of hemodialysis grafts after surgical thrombectomy. J Vasc Surg. 2012;55:472-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of SMART stent placement for arteriovenous graft salvage versus successful graft PTA. J Vasc Interv Radiol. 2005;16:1619-26.
- [CrossRef] [PubMed] [Google Scholar]
- Angioplasty with stent graft versus bare stent for recurrent cephalic arch stenosis in autogenous arteriovenous access for hemodialysis: A prospective randomized clinical trial. J Vasc Surg. 2008;48:1524-1531.e2.
- [CrossRef] [PubMed] [Google Scholar]
- Role of stents in hemodialysis vascular access. J Vasc Access. 2018;19:341-5.
- [CrossRef] [PubMed] [Google Scholar]
- Ischemia from extrinsic compression of the brachial artery by a stent in the venous outflow of a brachio-basilic arterio-venous graft. Seminars in Dialysis. 2013;26 [cited October 24, 2023]. Available from: https://onlinelibrary.wiley.com/doi/10.1111/sdi.12070
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of infection risk following covered stent exclusion of pseudoaneurysms in prosthetic arteriovenous hemodialysis access grafts. J Vasc Interv Radiol. 2012;23:69-74.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and economic benefits of stent grafts in dysfunctional and thrombosed hemodialysis access graft circuits in the revise randomized trial. J Vasc Interv Radiol. 2019;30:203-211.e4.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-coated balloons for the dysfunctional vascular access: An evidence-based road map to treatment and the existing obstacles. Semin intervent Radiol. 2022;39:056-65.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Drug-coated balloons for dysfunctional dialysis arteriovenous fistulas. N Engl J Med. 2020;383:733-42.
- [CrossRef] [PubMed] [Google Scholar]
- paclitaxel-coated balloons for the treatment of symptomatic central venous stenosis in dialysis access: Results from a randomized controlled trial. J Vasc Interv Radiol. 2017;28:811-7.
- [CrossRef] [PubMed] [Google Scholar]
- Multicentre, randomised, blinded, control trial of drug-eluting balloon vs Sham in recurrent native dialysis fistula stenoses. J Vasc Access. 2019;20:260-9.
- [CrossRef] [PubMed] [Google Scholar]
- Drug coated balloon angioplasty in failing AV Fistulas: A randomized controlled trial. CJASN. 2018;13:1215-24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A multicenter randomized controlled trial indicates that paclitaxel-coated balloons provide no benefit for arteriovenous fistulas. Kidney Int. 2021;100:447-56.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of fish oil supplementation and aspirin use on arteriovenous fistula failure in patients requiring hemodialysis: A randomized clinical trial. JAMA Intern Med. 2017;177:184.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: A randomized controlled trial. JAMA. 2012;307:1809.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA. 2008;299:2164.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360:2191-201.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. J Am Soc Nephrol. 2003;14:2313-21.
- [CrossRef] [PubMed] [Google Scholar]
- Role of antiplatelet therapy in the durability of hemodialysis access. J Nephrol. 2018;31:603-11.
- [CrossRef] [PubMed] [Google Scholar]
- Low-intensity warfarin is ineffective for the prevention of ptfe graft failure in patients on hemodialysis: A randomized controlled trial. J Am Soc Nephrol. 2002;13:2331-7.
- [CrossRef] [PubMed] [Google Scholar]
- An immediate access dialysis graft designed to prevent needle-related complications: Results from the initial pre-clinical studies. J Vasc Access. 2020;21:328-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Use of a novel immediate access dialysis graft designed to prevent needle-related complications: A first-in-man case report. J Vasc Access. 2021;22:475-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- HeRO Graft: Indications, technique, outcomes, and secondary intervention. Semin intervent Radiol. 2022;39:082-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A comparison between the HeRO graft and conventional arteriovenous grafts in hemodialysis patients. Semin Dial. 2014;27:310-8.
- [CrossRef] [PubMed] [Google Scholar]
- A review on the hemodialysis reliable outflow (HeRO) graft for haemodialysis vascular access. Eur J Vasc Endovasc Surg. 2015;50:108-13.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular arteriovenous fistula creation—review of current experience. Diagn. 2022;12:2447.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Midterm results of percutaneous arteriovenous fistula creation with the ellipsys vascular access system, technical recommendations, and an algorithm for maintenance. J Vasc Surg. 2020;72:2097-106.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular proximal forearm arteriovenous fistula for hemodialysis access: Results of the prospective, multicenter novel endovascular access trial (NEAT) Am J Kidney Dis. 2017;70:486-97.
- [CrossRef] [PubMed] [Google Scholar]
- Two-year cumulative patency of endovascular arteriovenous fistula. J Vasc Access. 2020;21:350-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparison between surgical and endovascular hemodialysis arteriovenous fistula interventions and associated costs. J Vasc Interv Radiol. 2018;29:1558-1566.e2.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of post-creation procedures and costs between surgical and an endovascular approach to arteriovenous fistula creation. J Vasc Access. 2017;18:S8-14.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular versus surgical creation of arteriovenous fistula in hemodialysis patients: Cost-effectiveness and budget impact analyses. J Vasc Access. 2021;22:48-57.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tracking and therapeutic value of human adipose tissue-derived mesenchymal stem cell transplantation in reducing venous neointimal hyperplasia associated with arteriovenous fistula. Radiol. 2016;279:513-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Therapeutic effect of adipose derived mesenchymal stem cell transplantation in reducing restenosis in a murine angioplasty model. JASN. 2020;31:1781-95.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rationale and trial design of mesenchymal stem cell trial in preventing venous stenosis of hemodialysis vascular access arteriovenous fistula (MEST AVF Trial) Kidney360. 2021 Dec. ;2:1945-52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Periadventitial local drug delivery to target restenosis. Vasc Pharmacol. 2018;107:12-9.
- [CrossRef] [PubMed] [Google Scholar]
- Periadventitial drug delivery for the prevention of intimal hyperplasia following open surgery. J Control Release. 2016;233:174-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Periadventitial application of rapamycin-loaded nanoparticles produces sustained inhibition of vascular restenosis. He X, editor. PLoS ONE.. 2014;9:e89227.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- 1α,25-Dihydroxyvitamin D3 encapsulated in nanoparticles prevents venous neointimal hyperplasia and stenosis in porcine arteriovenous fistulas. JASN. 2021;32:866-85.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Early application of an intermittent pneumatic compression device is safe and results in proximal arteriovenous fistula enlargement. J Vasc Access. 2019;20:24-30.
- [CrossRef] [PubMed] [Google Scholar]
- The FACT : Use of a novel intermittent pneumatic compression device to promote pre-surgery arm vein dilation in patients with chronic renal failure. J Vasc Access 2021:112972982110573.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review and meta-analysis of preoperative interventions to support the maturation of arteriovenous fistulae in patients with advanced kidney disease. Nephrol Dial Transplant. 2023;38:2330-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An implanted blood vessel support device for arteriovenous fistulas: A randomized controlled trial. Am J Kidney Dis. 2020;75:45-53.
- [CrossRef] [PubMed] [Google Scholar]
- Multicenter European real-world utilization of VasQ anastomotic external support device for arteriovenous fistulae. J Vasc Surg. 2022;75:248-54.
- [CrossRef] [PubMed] [Google Scholar]
- Nitric oxide resistance reduces arteriovenous fistula maturation in chronic kidney disease in rats. Jourd’heuil D, editor. PLoS ONE. 2016;11:e0146212.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nitric oxide releasing nanomatrix gel treatment inhibits venous intimal hyperplasia and improves vascular remodeling in a rodent arteriovenous fistula. Biomater. 2022;280:121254.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








