Translate this page into:
Exposure to Mycophenolic Acid and Its Clinical Response in an Indian Pediatric Population with Nephrotic Syndrome
Corresponding author: Ratna Prabha, Department of Pharmacology and Clinical Pharmacology, Christian Medical College, Vellore, Tamil Nadu, India. E-mail: ratnananya@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Deepthi RV, Arumadi M, Eriyat V, Mathew SK, Mathew BS, Agarwal I, et al. Exposure to Mycophenolic Acid and Its Clinical Response in an Indian Pediatric Population with Nephrotic Syndrome. Indian J Nephrol. 2024;34:323-7. doi: 10.25259/ijn_390_22
Abstract
Background:
Children with nephrotic syndrome experience many side effects and frequent relapses when treated with steroids and other drugs. Mycophenolic acid (MPA) is one of the effective and least toxic drug for the treatment of nephrotic syndrome. This drug needs to be monitored for maximal efficacy and minimal toxicity. The therapeutic reference range for this drug is not established for the aforementioned patient population of Indian origin.
Materials and Methods:
In this observational study, children with nephrotic syndrome on mycophenolate mofetil were followed up for a minimum duration of three months. Following this, their clinical status (relapse/remission) was determined and the mycophenolate exposure was measured for over 12 hours.
Results:
A total of 34 participants were included, with 17 (50%) in relapse. Median MPA Area under the curve over 12 hours (AUC0–12h) (36.5 µg·h/ml) in the remission group differed significantly compared to that in the relapse group (17.2 µg·h/ml).
Conclusion:
Higher exposure to MPA AUC0–12h is associated with clinical remission of pediatric nephrotic syndrome.
Keywords
Mycophenolic acid
Nephrotic syndrome
Pediatrics
Therapeutic drug monitoring
Introduction
Childhood nephrotic syndrome treated with steroids experience a high relapse rate (60%).1 Many progress to steroid-dependent/-resistant nephrotic syndrome and develop adverse effects from prolonged steroid therapy.2 Therefore, alternative strategies, such as treatment with mycophenolic mofetil (MMF), are practiced widely. MMF is recommended at a dose of 1200 mg/m2 in two divided doses for 12.3 MMF is the ester prodrug of mycophenolic acid (MPA).2,4
A prospective crossover trial, performed in children with nephrotic syndrome, observed a lower relapse rate in those who had high MPA Area under the curve over 12 hours (AUC0–12h) ≥ 50 μg·h/ml compared to those having MPA AUC0–12 h < 50 μg·h/ml.5 There are other studies that propose a lower MPA AUC0–12h (as low as a value ≥30 μg·h/ml), which may be effective in treating nephrotic syndrome.6,7 Nevertheless, these studies show that a higher exposure to MPA is required to prevent a relapse of the disease. All of these studies5-7 used only three blood samples to determine the 12-hour MPA AUC.
Therapeutic drug monitoring (TDM) of MPA is not a standard of care. The difference in recommended targets for MPA AUC could be a reason for the lack of integration of TDM into routine clinical practice. In this study, a full inter-dose (12-hour) concentration-time profile of MPA was estimated, and the exposure in patients who relapsed and who were under remission was compared with the final goal of proposing a target MPA AUC0–12h for preventing relapses in pediatric patients with nephrotic syndrome.
Materials and Methods
This was an observational study performed on children from 2–17 years, who were diagnosed with frequently relapsing nephrotic syndrome and who attended the pediatric nephrology outpatient department (OPD) from October 2019 to October 2020. The Institutional Review Board (IRB) of Christian Medical College, Vellore (Min No. 9085) approved the study protocol. Written and informed consent was obtained from the parents, and assent from the participants was obtained, wherever applicable.
The dose of MPA administered ranged from 600 to 1200 mg/m2 per day (graded according to their clinical response), and all of them were on MMF (Ipca Laboratories) for a minimum period of three months. A drug history diary was maintained. Pill count was done to ensure compliance. Participants were excluded from the study if they showed poor compliance to the treatment, had an infection-induced relapse, or had diarrhea. We excluded patients with infection-induced relapse due to their intake of antibiotics, which could affect the exposure to MPA. The status of the patient with regard to remission/relapse at the time of recruitment was evaluated clinically and biochemically in the outpatient department, and other demographic data were collected.
On the day of the test, anthropometric measurements were taken; overnight fasting status and time of last dose were confirmed. A dedicated intravenous access was obtained, and the trough blood sample (2 ml) was collected in K2EDTA tube followed by administration of the dose of the MMF under direct observation. Further blood samples (2 ml) were collected at 0.5, 1.0, 1.5, 2.0, 2.5, 3, 4, 6, 8 and 12 hours post dose. The samples were centrifuged, and the plasma was separated and kept in a freezer (at −20°C) until analysis by a high-performance liquid chromatography (HPLC) UV detector. Chromatographic response for MPA and internal standard carbamazepine were measured at 254 nm UV wave length.
Participants were instructed to avoid any medication or food for three hours post ingestion of MMF. The details regarding the dosage of prednisolone and other concomitant drugs and history of relapse/remission were also collected.
The MPA AUC0–12h was calculated using the trapezoid method. The correlation between MPA trough and MPA AUC0–12h was determined. The median MPA AUC0–12h was estimated in patients who relapsed and was compared (Wilcoxon rank-sum test) to patients under remission. Data analysis and visualizations were performed using R software (R version 4.1.3). The receiver operator characteristic (ROC) cutoff was determined with 100% sensitivity, as our intention was to identify a cutoff to prevent relapse in 100% of patients.
Calibrators, quality control, extraction method, and HPLC conditions
MPA concentrations in plasma were measured using a validated HPLC (proficiency tested by the Royal College of Pathologists of Australasia). The stock solutions of mycophenolic acid (≥98%, Sigma), calibrators (30 µg/ml, 10 µg/ml, 1 µg/ml), and two quality control (QC) samples of 5 µg/ml and 25 µg/ml were prepared in HPLC-grade acetonitrile (ACN). Carbamazepine was used as the internal standard (IS), prepared by diluting the stock solutions using plasma. We followed a simple extraction method for protein precipitation by using ACN, and the supernatant was separated by centrifugation at 13,000 rpm for seven minutes; 20 µl of the supernatant was injected. We used the LC-2010A HT liquid chromatograph system (Shimadzu, Japan) with C18 column (Shimadzu ShimPak GIST, 4.6 × 250 mm, 5-µm particle size). The mobile phase consisted of 20 mM phosphate buffer (K2HPO4 and KH2PO4, pH: 3.5) and ACN (Qualigens, Thermo-Fischer) in 49:51 ratio at a flow rate of 1.2 ml/min. Quantification of MPA and IS was performed at 254 nm. The chromatogram peak area ratio of MPA and IS was used for calculating concentrations at each time point.
Results
Our study population (n = 34) consisted predominantly of male children (n = 23, 62%) and the mean age was 109 ± 43.63 months. The duration of illness ranged from 8 to 148 months, with the median duration of MMF therapy being 11 months. One participant was excluded from the analysis as the trough concentration was very high and the timing of last drug dose intake was inappropriate. Seventeen participants (50%) had relapsed at the time of MPA AUC0–12h estimation. Most of the children (n = 29/34, 85%) had relapsed previously at some point during their illness and many (n = 12/34, 35%) had experienced more than one relapse [Table 1]. The children who were experiencing relapse or recently had a relapse were receiving oral prednisolone. The other medications received included proton pump inhibitors, antihypertensive drugs (enalapril and/or losartan), and atorvastatin.
| Characteristics | Summary statistics (n = 34) |
|---|---|
| Gender | Female: 13 (38%), Male: 21 (61.7%) |
| Age (months) | 101 (48-184) |
| No. of patients with current relapse | 17 (50%) |
| No. of patients with previous relapse | 29 (85%) |
| Previous number of relapses (median [range]) | 1 (0-5) |
| Duration of illness in months (median [range]) | 57.5 (8-148) |
| Dose of MPA in mg/kg (median [range]) | 33.2 (16.9-61.8) |
| Dose of MPA in mg/m2 (median [range]) | 917 (423-1600) |
| Prednisolone daily dose in mg (median [range]) | 3.75 (0-60) |
| Serum creatinine in mg/dl (median [range]) | 0.32 (0.12-0.53) |
| Serum albumin in mg/dl (median [range]) | 3.45 (1.3-4.6) |
MPA: Mycophenolic acid.
The MPA trough concentrations ranged from 0.00 to 3.62 µg/ml (median: 1.19 µg/ml) and the peak (Cmax) concentration ranged from 5.84 to 35.8 µg/ml. Time of peak concentration (Tmax) was 0.5 h for most of the participants (n = 22/35, 63%) [Figure 1]. The MPA AUC0–12h ranged from 6.8 to 96.3 µg·h/ml (median: 25.6 µg·h/ml). The Pearson correlation coefficient (r) between morning trough and evening trough was 0.62. Inter-individual variability (coefficient of variation, CV%) for morning trough, AUC0–12h, and dose normalized AUC0–12h was 82%, 59%, and 53%, respectively. The Pearson correlation coefficient (r) between total dose and MPA AUC0–12h was 0.21.
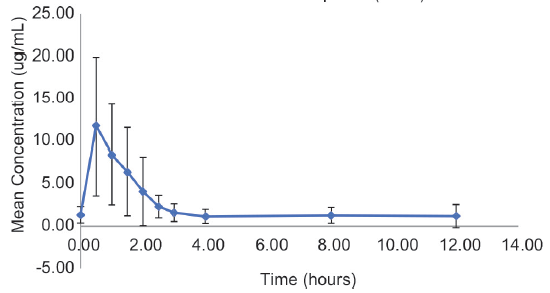
- Mean concentration-time profile of pediatric patients with nephrotic syndrome. The error bar plotted is one standard deviation.
Of the total number of patients, 18 (51%) had MPA AUC0–12 h < 30 µg·h/ml, with a median (range) MMF dose of 860 mg/m2 (423–1428), whereas 16 had MPA AUC0–12 h > 30 µg·h/ml, with a median (range) MMF dose of 1048 mg/m2 (600–1600).
The median MPA AUC0–12h (36.45 µg·h/ml) in the remission group was significantly different from that in the relapse group (median: 17.2 µg·h/ml; Wilcoxon rank-sum test, P < 0.001) [Figure 2]. The trough and peak MPA concentrations were also significantly different among those with remission and relapse [Table 2]. The Tmax was similar in these two groups (Wilcoxon rank-sum test, P = 0.17). An ROC cutoff for AUC0–12h of 48 µg·h/ml was identified with a sensitivity of 100%.
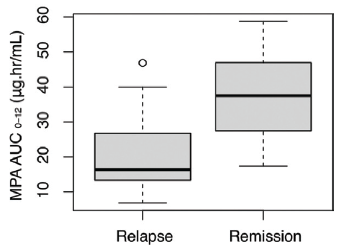
- MPA AUC0–12h between relapse and remission. MPA AUC0-12h: Mycophenolic acid area under the curve over 12 hours
| Parameter (median [range]) | Participants under relapse (n = 17) | Participants under remission (n = 17) | *P |
|---|---|---|---|
| MPA AUC0–12 h (μg·h/ml)** | 17.2 (13.7-24.5) | 37.0 (26.0-46.0) | <0.001 |
| MPA trough (μg/ml) | 0.8 (0.7-1.2) | 1.5 (0.9-2.7) | 0.03 |
| MPA Cmax (μg/ml) | 8.6 (4.2-13.9) | 17.0 (10.8-22.3) | <0.01 |
| MPA dose (mg/kg) | 35.7 (33.1-42.9) | 31.5 (24.2-36.6) | 0.13 |
| Serum creatinine | 0.3 (0.2-0.4) | 0.3 (0.3-0.4) | 0.26 |
| Serum albumin | 2.4 (1.7-2.8) | 3.9 (3.5-4.1) | <0.001 |
| Serum protein | 4.6 (4.2-5.5) | 6.5 (6.1-6.7) | <0.001 |
| Frequency of relapses | 0.23 per month | 0.10 per month | <0.001 |
Discussion
In this study, there was no significant correlation between total dose or normalized dose with MPA AUC (0.25 and 0.21, respectively). This finding was in agreement with a study done by Tellier et al.8 The dose of MMF was not statistically different in patients under relapse or remission. However, there was a statistically significant difference in MPA AUC0–12h between these groups. This demonstrates the lack of concordance between the MMF dose and MPA AUC0–12h. In our study, interpatient variability of trough concentration and AUC0–12h was high, as observed in various studies.9–11
Our study is in agreement with various studies published in western literature like that of Gellerman et al.,5 who proposed a cutoff of MPA AUC0–12h (57.1 µg·h/ml) for predicting clinical outcome when treating patients with nephrotic syndrome. Tong et al.6 suggested a cutoff of 27.99 µg·h/ml with a sensitivity of 65.2% and specificity of 87.5% for differentiating relapsing patients from non-relapsing ones. They also showed that the steroid dose requirement and number of relapses were low in the high AUC0–12h group, with MPA AUC being ≥30 µg·h/ml at the one-month visit. A study published by Chen et al.12 on systemic lupus erythematosus (SLE) pediatric patients also recommended an AUC0–12h of more than 50 mg·h/L for disease control. Hackl et al.7 demonstrated that an MPA AUC0–12h lower than 44.6 µg·h/ml could be used to differentiate relapse from non-relapse in nephrotic syndrome patients, and thus suggested that an exposure above this value may be indicated to decrease relapse frequency. In our study, none of patients with an MPA AUC0–12 h > 48 µg·h/ml were in relapse. We did not observe any adverse effect toward MPA among the patients we recruited for study. Therefore, we propose a high MPA AUC0–12h cutoff of that is >48 µg·h/ml as a reliable target to prevent relapse among pediatric patients with nephrotic syndrome. This target should be confirmed for its clinical efficacy in future clinical trials. In our study, we recruited patients with a minimum follow-up period of three months. Thus, the suggested target MPA AUC0–12h should be evaluated for its clinical efficacy for longer periods of treatment with MPA.
MPA is highly protein-bound (>95%),13 and, therefore, serum albumin level is an important confounding factor in total systemic MPA exposure. Theoretically, as albumin concentration decreases the unbound or free fraction of MPA increases, thereby leading to increased MPA availability for metabolism and elimination.13 In this study, the serum albumin concentration of patients under relapse were lower than those under remission. This may partially explain the significantly lower MPA exposure in patients who relapsed.
MPA AUC0–12h is a useful tool in therapeutic drug monitoring (TDM). However, measuring the total AUC0–12h is expensive, time-consuming, and requires multiple samplings over the inter-dose interval. Moreover, no specific MPA AUC0–12h target range has been established for children with nephrotic syndrome. This has led to increased resistance among the health-care community to perform TDM in pediatric populations. We attempted the validation of a limited sampling strategy (LSS) equation that was developed for pediatric SLE patients.9 However, the equation failed to accurately predict MPA AUC in nephrotic syndrome patients. Subset regression analysis was done for the current data, and two LSS equations were developed with adjusted R2 > 0.94 to predict the observed AUC0–12h. Both equations used five time points of blood collection, with Equation 1 needing collection time points of up to eight hours post dose and Equation 2 needing only up to three hours post dose. These LSS equations could be used to estimate AUC0–12h in patients having nephrotic syndrome with reasonable accuracy and could thus be used for dose optimization in children with nephrotic syndrome [Figures 3 and 4].
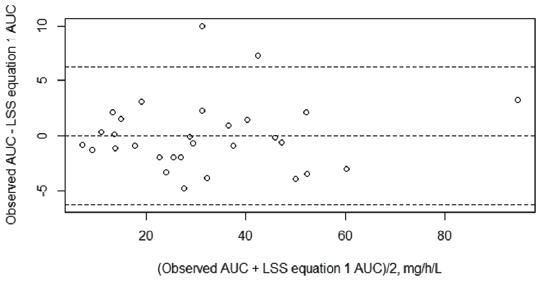
- Bland–Altman plot for limited sampling strategy 1. AUC: Area under the curve; LSS: Limited sampling strategy.
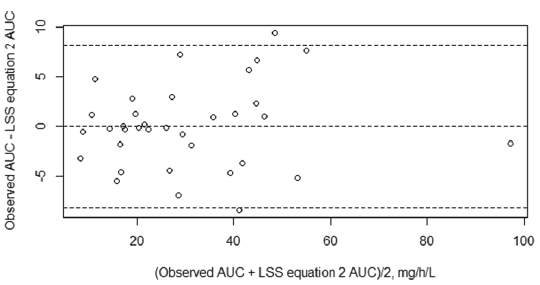
- Bland–Altman plot for limited sampling strategy 2. AUC: Area under the curve; LSS: Limited sampling strategy.
Limited sampling strategy (LSS) equation No. 1
−1012115 + 1.9044C0 + 0.45780C0.5 + 0.63719C1 + 4.20119C2.5 + 5.19074C8
Limited sampling strategy (LSS) equation No. 2
3.10096 + 4.47209C0 + 0.72995C1 + 0.66457C1.5 − 0.7733C2 + 5.7379C3
In future, prospective clinical studies, including randomized control trials, should be performed to determine the efficacy of targeting an MPA AUC0–12h of 48 µg·h/ml in preventing relapse among pediatric patients with nephrotic syndrome. This will help to negate the effects of confounding factors such as serum albumin in determining the clinical efficacy of targeting 48 µg·h/ml in preventing relapse of nephrotic syndrome.
Conclusion
Higher MPA (AUC 0 - 12) is associated with remission of pediatric nephrotic syndrome.
Acknowledgments
We acknowledge the study participants and their parents for their willingness to take part in this intensive blood sampling study. We also acknowledge our technical staff Ms. Lavanya, Mr. Hassain for analyzing patient samples.
Financial support and sponsorship
We received funding through the internal fluid research grant from Christian Medical College, Vellore.
Conflicts of interest
There are no conflicts of interest.
References
- Mycophenolate mofetil therapy in frequently relapsing steroid-dependent and steroid-resistant nephrotic syndrome of childhood: Current status and future directions. Pediatr Nephrol. 2005;20:1376-81.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric idiopathic nephrotic syndrome: Treatment strategies in steroid dependent and steroid resistant forms. Curr Med Chem. 2010;17:847-53.
- [CrossRef] [PubMed] [Google Scholar]
- KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1-276.
- [CrossRef] [PubMed] [Google Scholar]
- Katzung BG, ed. Basic and Clinical Pharmacology (14th ed). New York Chicago San Francisco Athens London Madrid Mexico City Milan New Delhi Singapore Sydney Toronto: McGraw-Hill Education; 2018. p. :1250. (A Lange medical book)
- Mycophenolate mofetil versus cyclosporin a in children with frequently relapsing nephrotic syndrome. JASN. 2013;24:1689-97.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The value of monitoring the serum concentration of mycophenolate mofetil in children with steroid-dependent/frequent relapsing nephrotic syndrome. Nephron. 2016;132:327-34.
- [CrossRef] [PubMed] [Google Scholar]
- Mycophenolate mofetil therapy in children with idiopathic nephrotic syndrome: Does therapeutic drug monitoring make a difference? Ther Drug Monit. 2016;38:6.
- [CrossRef] [PubMed] [Google Scholar]
- Mycophenolic acid pharmacokinetics and relapse in children with steroid–dependent idiopathic nephrotic syndrome. Clin J Am Soc Nephrol. 2016;11:1777-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Development and validation of limited sampling strategy equation for mycophenolate mofetil in children with systemic lupus erythematosus. Indian J Nephrol. 2016;26:408-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- To what extent does the understanding of pharmacokinetics of mycophenolate mofetil influence its prescription. Pediatr Nephrol. 2004;19:962-5.
- [CrossRef] [PubMed] [Google Scholar]
- High interpatient variability in response to mycophenolic acid maintenance therapy in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 2015;30:i138-45.
- [CrossRef] [PubMed] [Google Scholar]
- PK/PD study of mycophenolate mofetil in children with systemic lupus erythematosus to inform model-based precision dosing. Front Pharmacol. 2020;11:605060.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pharmacokinetic modelling of the plasma protein binding of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet. 2009;48:463-76.
- [CrossRef] [PubMed] [Google Scholar]







