Translate this page into:
Favorable outcome in atheroembolic renal disease with pulse steroid therapy
Address for correspondence: Dr. Alok Sharma, Senior Consultant, Department of Histopathology, National Reference Laboratory, Dr. Lal Path Labs Pvt. Ltd., Block E Sector 18, Rohini, New Delhi - 110 085, India. E-mail: dr_aalok@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Atheroembolic renal disease is characterized by renal failure secondary to occlusion of renal vasculature by cholesterol containing atheromatous plaques. Clinical presentations of this disease entity are myriad, with limited therapeutic options and unfavorable outcomes. This report describes an elderly male patient with peripheral vascular disease who developed acute renal failure during hospital admission for rectal bleed, and was diagnosed with atheroembolic renal disease on renal biopsy. The patient was managed with pulse steroid therapy and had a favorable outcome.
Keywords
Atheroembolic renal disease
pulse steroid therapy
renal biopsy
Introduction
Atheroembolic renal disease (AERD) is defined as renal failure secondary to the occlusion of renal vasculature by cholesterol containing atheromatous plaques dislodged from the aorta or other major arteries.[1] This occurs either spontaneously, or following invasive vascular procedures such as angiographic catheter insertions, or after the use of anticoagulant and thrombolytic agents.[2] AERD is often not suspected clinically, and mimics as well as coexists with several other systemic diseases.[3] In spite of advances in imaging modalities and clinical experience with this entity since its first description in 1862,[4] several issues regarding its diagnosis and management still remain unresolved.
Case Report
A 73-year-old-man with 40 pack years of smoking history and hypertension was on treatment with amlodipine 5 mg per day. He was diagnosed with peripheral vascular disease when a surgical consultation for purplish discoloration of toes was sought 3 months ago [Figure 1a] and warfarin sodium 5.0 mg alternating with 2.5 mg per day was prescribed.

- (a) Clinical photograph of the patient showing the purplish discoloration of the toes. (b) Photomicrograph of renal biopsy showing the characteristic biconcave needle shaped cholesterol atheroemboli lodged in an interlobular sized artery (H and E, ×400)
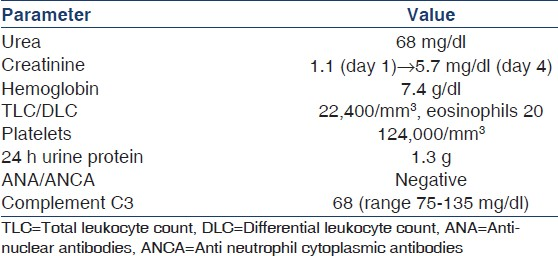
In the current admission, he presented with passage of black stools and fresh bleeding per rectum. Blood pressure at admission was 150/90 mmHg. There were no clinical features of dehydration or excessive fluid loss. Warfarin was stopped and an upper gastrointestinal endoscopic examination was performed, which showed a healed duodenal ulcer. Abdominal ultrasound examination revealed an abdominal aortic aneurysm. Renal artery Doppler study was normal. Urine output was adequate on the first two days of admission (about 1800 ml) and urine examination at this time showed trace proteinuria, 8-10 pus cells/hpf. There was no evidence of hematuria or heme granular casts on microscopic examination of urine sediment. On the third admission day, he developed abrupt swelling of both legs and sharp decline in urine output to 600 ml which further dropped to 400 ml on fourth day, with development of azotemia. The relevant laboratory parameters at presentation are depicted in Table 1. Broad spectrum antibiotics were administered and hemodialysis was initiated. Pending the results of serological investigations, a clinical suspicion of vasculitis was considered and three doses of injection methyl prednisolone 1 gm intravenous daily for three days were administered starting from fifth admission day, followed by oral prednisolone 60 mg per day from 4th day. Inj. cyclophosphamide 750 mg (i.v.) was given on 8th post admission day. A renal biopsy was meanwhile performed which revealed presence of biconvex needle shaped empty spaces in lumen of an interlobular sized artery, consistent with cholesterol atheroemboli [Figure 1b]. Tubular atrophy and interstitial fibrosis involved about 40% of the cortex, and the proximal tubules in the viable areas showed features of acute tubular injury, including loss of brush borders, epithelial simplification luminal granular casts. Direct immunofluorescence examination was negative for immunoglobulins (IgA, IgG, IgM), complements (C3, C1q), and also for kappa and lambda light chains. No refractile crystals were noted in frozen sections of biopsy core submitted for Direct Immunofluoroscence (DIF) studies. A final diagnosis of renal failure secondary to atheroembolic renal disease possibly precipitated by oral anticoagulant therapy was rendered.
Patient's clinical status gradually improved with progressive increase in urine output and reduction of serum creatinine to 2.3 mg/dl on 21st post admission day [Figure 2]. After review of kidney biopsy report, the oral prednisolone was tapered rapidly in 2 weeks, and cyclophosphamide was withdrawn. Hemodialysis was discontinued and patient was discharged, off dialysis in a clinically stable condition with advice for regular outpatient follow-up.
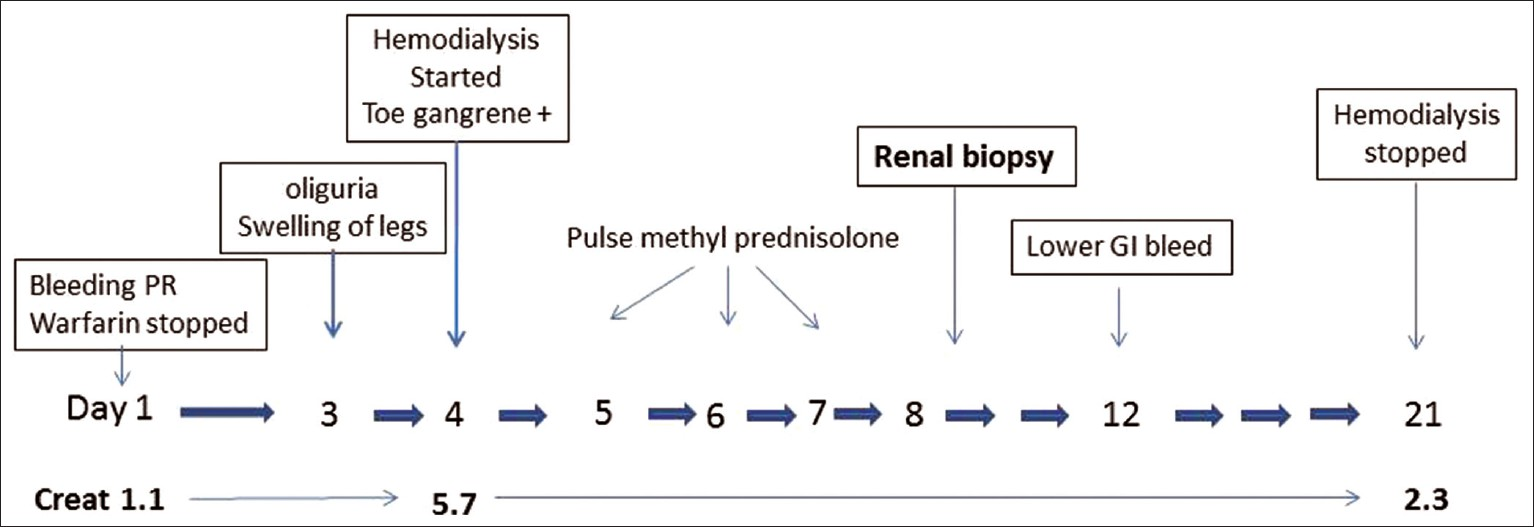
- Image depicting the clinical course of disease
Discussion
AERD is a part of systemic disease triggered by the release of cholesterol emboli from a disrupted atheromatous plaque usually located in the aorta to several organs. Renal involvement is frequent owing to their anatomical proximity to the aorta and high rate of perfusion.
Incidence of AERD is difficult to estimate as it is often underdiagnosed owing to its propensity to coexist with as well as mimic several other renal disorders.[3] The reported incidence in biopsy series varies from 1.1% to 4.5% in various studies[5] and an approximately similar incidence in few autopsy studies.[6]
The classic clinical manifestations of AERD include live do reticularis (purplish rash over lower extremities and abdominal wall), purple toes, small nail bed infarcts and various other manifestations related to lodgement of atheroemboli in other organs such as muscles (myalgia), joints (arthralgias), pancreas (acute pancreatitis) gastrointestinal tract (perforation) or the eye (retinal infarction). It is worth noting that many of these manifestations are also associated with systemic vasculitis, which is often the first clinical differential diagnosis in patients with AERD,[3] as in the present case. A further diagnostic difficulty arises in patients with pre-existing peripheral vascular disease, wherein livideo reticularis may be mistaken for manifestations of peripheral vascular disease, leading to a delay in diagnosis of the condition. In the present case, the finding of live do reticularis (purplish discoloration of lower extremities and toes), was ascribed to peripheral vascular disease, and oral anticoagulants were prescribed, which possibly led to the sequence of events culminating in AERD.
AERD has a variable, but definite temporal relationship to invasive vascular procedures and use of thrombolytic and anticoagulant therapy.[2] Overall, it is estimated that about 30-85% of patients with AERD have a history of invasive vascular procedure in the preceding 3 months. Studies have shown that about 13-22% patients with AERD received anticoagulant therapy (heparin or warfarin),[1] as noted in the present case. The mechanism involves dissolution of superficial clots which destabilize the ruptured atherosclerotic plaques, resulting in showering of cholesterol atheroemboli into the circulation.
The gold standard of diagnosing AERD remains the demonstration of atheroemboli in renal vasculature in a renal biopsy. Delay or failure to diagnose this condition often stems from hesitation to perform renal biopsies, as the patients are elderly and have compromised kidneys due to co-existing morbidities. Cholesterol emboli appear as negative biconvex shadows appearing as “ghosts”. Involvement of renal vasculature is patchy, and the diagnosis may be missed if few sections are examined in a biopsy. In the present case also the emboli were visualized in only three of the 43 sections examined, and were also absent from the tissue submitted for immunofluorescence examination.
Management of AERD is still empirical and no defined protocols have been developed. Surgical management includes repair of aortic aneurysms, interposition of prosthetic bypass graft, angioplasty or extracorporeal arterial reconstruction.[7] Medical management involves use of statins[8] or corticosteroids. Few studies have demonstrated the benefits of pulse steroid therapy in patients with AERD.[9–12] We noted a steady improvement in patient's clinical condition and renal parameters following pulse steroid therapy. Although successful treatment of AERD using both high (intravenous pulse steroids)[9] and low dose (0.3 mg/kg/day)[10] corticosteroids has been reported, a careful approach is necessary as both these therapeutic options may predispose the patients (who frequently suffer from significant co-morbidities) to infectious complications. The rationale behind use of steroids in AERD is not clear, but may be related to the suppression of reactive inflammation often seen along with the atheromatous plaques. Activation of the complement (C5) in vitro along with evidence of hypocomplementemia and eosinophilia in many patients suggest a possible role for inflammation in pathogenesis of AERD and, perhaps the response to steroid therapy.[1]
Outcome of AERD is generally considered poor with high mortality rate. In a large study, older age, comorbid conditions (presence of diabetes and history of heart failure), baseline renal dysfunction, time course of renal function decline, and extra renal manifestations were associated with a significant increased risk for both ESRD and death.[13]
AERD remains an enigmatic disease often underdiagnosed and misunderstood. A greater awareness regarding its clinical manifestations is essential and in the relevant clinical setting, a timely performed renal biopsy can allow early diagnosis and initiation of appropriate treatment. Pulse steroid therapy appears to be an effective modality and may be offered as a first line treatment in patients with AERD.
Source of Support: Nil
Conflict of Interest: None declared
References
- Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150:1237-42.
- [Google Scholar]
- Vasculitis look-alikes: Variants of renal atheroembolic disease. Nephrol Dial Transplant. 1999;14:430-3.
- [Google Scholar]
- Experimentelle Beitragezur Lehre von der Embolie. Virchows Arch Pathol Anat Physiol. 1862;25:308-10.
- [Google Scholar]
- Renal biopsy in patients 65 years of age or older.An analysis of the results of 334 biopsies. J Am Geriatr Soc. 1990;38:669-74.
- [Google Scholar]
- Atheromatous emboli to the kidneys after aortic surgery. N Engl J Med. 1957;257:442-7.
- [Google Scholar]
- Improvement in renal cholesterol emboli syndrome after simvastatin. Lancet. 1998;351:1331-2.
- [Google Scholar]
- Cholesterol crystal embolization (CCE): Improvement of renal function with high-dose corticosteroid treatment. Saudi J Kidney Dis Transpl. 2011;22:327-30.
- [Google Scholar]
- The effect of low-dose corticosteroids on short- and long-term renal outcome in patients with cholesterol crystal embolism. Ren Fail. 2011;33:298-306.
- [Google Scholar]
- Cholesterol crystal embolization mimicking vasculitis: Success with corticosteroid and cyclophosphamide therapy in two cases. Rheumatol Int. 2006;26:454-60.
- [Google Scholar]
- Cholesterol embolization treated with corticosteroids-two case reports. Angiology. 2005;56:497-501.
- [Google Scholar]
- The challenge of diagnosing atheroembolic renal disease: Clinical features and prognostic factors. Circulation. 2007;116:298-304.
- [Google Scholar]







