Translate this page into:
Fibroblast growth factor-23 levels in maintenance hemodialysis patients in India
Address for correspondence: Dr. U. Anandh, Department of Nephrology, Yashoda Hospitals, Secunderabad, Telangana, India. E-mail: uanandh@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Fibroblast growth factor-23 (FGF-23) levels start rising early in patients with chronic kidney disease and is implicated in cardiovascular and overall mortality of hemodialysis patients. We conducted a prospective observational cohort study in stable dialysis patients looking into the levels of FGF-23 in hemodialysis patients and its association with various demographic and biochemical variables and mortality. A total of 91 patients were enrolled in the study. The mean FGF-23 levels were very high (1152.7 pg/ml). FGF-23 levels were significantly associated with serum phosphorus and parathyroid hormone (PTH) levels in univariate and multivariate analysis. No significant association between FGF-23 and cardiovascular comorbidities and overall mortality was seen. FGF-23 levels rise exponentially in maintenance hemodialysis patients. There is a strong association between FGF-23 and phosphorus and PTH levels. No association between FGF-23 and mortality was noted in our patients.
Keywords
Fibroblast growth factor-23
hemodialysis
mortality
parathyroid hormone
phosphorus
Introduction
Fibroblast growth factor-23 (FGF-23) is a hormone secreted by the bone cells - osteocytes and osteoblasts.[1] It is increasingly recognized as intimately connected to the uremic state and its complications. Its levels increase as the stage of chronic kidney disease (CKD) advances,[2] reaching very high levels in CKD Stage 5. It is also believed to be involved in cardiovascular and endothelial dysfunction in CKD.[3]
Many studies over the last decade have reported FGF-23 as a factor of prognostic significance in CKD.[4] Very little data about the significance of this biomarker exists in Indian scientific literature and our study report the results of the levels of FGF-23 in stable maintenance hemodialysis patients.
Materials and Methods
All patients who were continuing in our maintenance hemodialysis program in January 2012 were enrolled as a prospective observational cohort. This group was longitudinally followed for 2 years till January 2014.
At the beginning of the study, the demographic profile, native kidney disease, and comorbidities were noted. Left ventricular hypertrophy and ischemic heart disease where defined based on standard criteria. The dialysis details were also recorded. The hematochemical parameters and serum FGF-23 levels were tested at the beginning of the study. Routine hematological and biochemical parameters were tested as per dialysis protocols.
FGF-23 levels were measured in our biochemistry laboratory using the kit EZHFGF-23-32 K human FGF-23 Elisa kit (Merck–Millipore Corporation, Billerica, MA 01821, USA). It is a colorimetric fluorescent assay. For the analysis of the association of FGF-23 with various studied variables, the levels were divided into two groups (Group I, FGF 23 <300 pg/ml and Group II FGF-23 ≥300 pg/ml). All statistical analysis was performed using the Statistical Software for windows version 20.0 SPSS Version 20.0 (IBM Corp. Armonk, NY, 2011).
Results
A total of 91 patients were enrolled in the cohort study. No patient dropped out of the study.
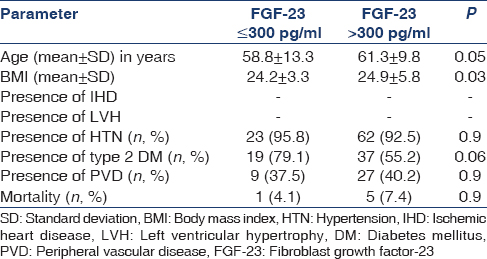
The mean ± standard deviation (SD) age of 91 patients (females 34) was 60.6 ± 10.8 years. During follow-up, in the next 48 months, five patients were transplanted and are doing well. Two patients were shifted to peritoneal dialysis who are also alive and healthy until till the end of the study period. There were six deaths during the study period. Fifty-six of these patients had type 2 diabetes mellitus as the cause of their CKD. The body mass index (BMI) of the group is shown in Table 1a.

The mean ± SD duration of dialysis in months was 47.2 ± 24.8 months. Majority of patients were on thrice weekly dialysis [Table 1b].

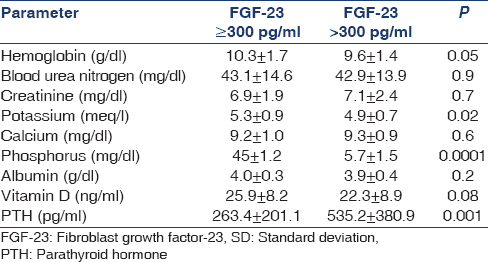
The mean ± SD and the range of the major biochemical and hematological parameters are given in Table 2. The serum calcium and phosphorus levels were reasonably well controlled. Serum parathyroid hormone (PTH) level was consistently high, and the Vitamin D level was low in general for the whole cohort.

FGF-23 level was high in the whole cohort. There was a wide range of FGF-23 levels in this group. The mean FGF-23 level was 1152.7 pg/ml. Patients with higher FGF-23 levels tend to be older, have higher BMI and on dialysis for a shorter duration [Table 3a]. The frequency of dialysis and the FGF-23 levels had no significant association. Type 2 diabetes mellitus was more common in the Group II. No significant association was noted with cardiovascular comorbidities and mortality [Table 3b].

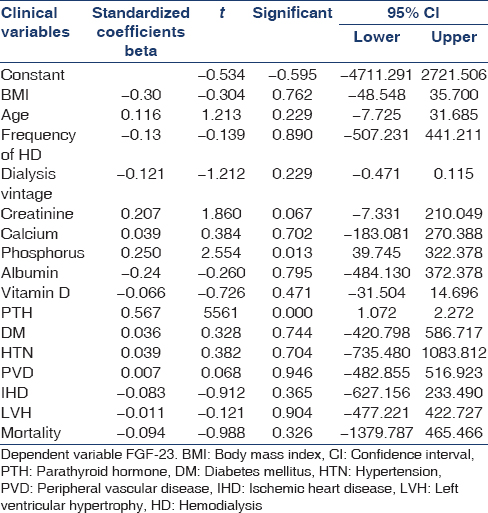
There was a strong association between FGF-23 and serum phosphorus levels and PTH levels. There was a trend to an inverse relationship with FGF-23 and Vitamin D levels [Table 4]. A multivariate analysis confirmed the results noted in the univariate analysis [Table 5].
Discussion
Patients with CKD have raised serum FGF-23 levels. These levels start rising in early stages of CKD and have an exponential increment in levels in stage 5 CKD, especially patients on dialysis.[5]
FGF-23 levels rise with rising serum phosphorus levels. Rising FGF-23 levels suppress 1,25-hydroxy Vitamin D levels.[6] The rise in PTH correlated with FGF-23 in CKD patients and this is attributed to the suppressive effect of FGF-23 on Vitamin D levels.[7]
FGF-23 level was independently associated with a higher risk of myocardial infarction, stroke, coronary, carotid, and lower limb revascularization, lower extremity amputation and death.[8] In some studies, a significantly higher rate of congestive heart failure in patients with higher FGF-23 levels was noted.[9]
Our study re-emphasizes the strong association between serum phosphorus and PTH levels in both univariate and multivariate analyses. Simple statistical analyses (both univariate and multivariate) did not show any link between FGF-23 and cardiovascular comorbidities (both the presence of left ventricular hypertrophy and ischemic heart disease). There was also no link between FGF-23 and mortality. This lack of association has been seen in a recent study also.[10]
The strength of our study is that it is the first of its kind which addresses the issue of FGF-23 levels in relativelyhomogenous stable hemodialysis patients.
The limitation of our study is that it is a relatively small study with a short duration (2 years) follow-up. The cohort of patients also is not fully representative of dialysis patients in India as they came from a relatively higher socioeconomic background.
Conclusion
There is a strong positive association between FGF-23, phosphorus and PTH levels. FGF-23 levels in our study continue to remain high despite acceptable control of phosphorus. Our study did not show any link between FGF-23 and cardiovascular morbidity and overall mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500-5.
- [Google Scholar]
- Fibroblast growth factor 23 in chronic kidney disease: Bridging the gap between bone mineral metabolism and left ventricular hypertrophy. Blood Purif. 2011;31:26-32.
- [Google Scholar]
- Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584-92.
- [Google Scholar]
- Residual renal function is an independent determinant of serum FGF-23 levels in dialysis patients. Nephrol Dial Transplant. 2012;27:2017-22.
- [Google Scholar]
- Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21:1427-35.
- [Google Scholar]
- Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011;80:475-82.
- [Google Scholar]
- Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432-9.
- [Google Scholar]
- FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant. 2010;25:3983-9.
- [Google Scholar]
- Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J. 2011;161:956-62.
- [Google Scholar]







