Translate this page into:
Flow Cytometry as a Diagnostic Tool in Monoclonal Gammopathy of Renal Significance
Address for correspondence: Dr. Jasmita Dass, Department of Hematology, All India Institute of Medical Sciences, Room No. 206, AIIMS, New Delhi, India. E-mail: jasmita@aiims.edu, drjasmita@gmail.com
-
Received: ,
Accepted: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Dear Editor,
Renal diseases associated with monoclonal gammopathy without symptomatic multiple myeloma (MM), Waldenstrom’s macroglobulinemia (WM), or chronic lymphocytic leukemia (CLL) are increasingly known.[1] Many of these patients have a small clonal population of plasma cells (PCs) or B cells. The International Kidney and Monoclonal Gammopathy Research group (IKMG) introduced the term monoclonal gammopathy of renal significance (MGRS) in 2012.[1] The MGRS includes monoclonal gammopathy of uncertain significance (MGUS), smoldering MM, smoldering WM, monoclonal B-cell lymphocytosis (MBL), CLL, and low-grade B-NHL associated with renal involvement.[12] The diagnosis of MGRS is based on renal biopsy and monoclonal protein identification. B-cell or PC clone identification is paramount for a clone-directed therapy for long-term hematologic response.[2] As these clones are small, a highly sensitive technique like flow cytometry (FCM) should be used to identify clonality.[3] It is important to identify MGRS as these patients do not respond well to immunosuppressive therapy, have a high rate of recurrence post renal transplantation, and can progress to corresponding hematological malignancy.[4]
We are describing two cases of MGRS where we could confirm the presence of a small clonal PC population using FCM. The case characteristics are listed in Table 1.
| Case 1 | Case 2 | |
|---|---|---|
| Age in years/sex | 55/Female | 49/Male |
| Renal biopsy | C3 glomerulopathy | Monoclonal immunoglobulin deposition disease (IgG lambda) |
| Clinical symptoms | Bilateral lower limb swelling, periorbital swelling, hematuria and hypertension, transfusion-dependant anemia | Periorbital swelling, hypertension, progressive renal dysfunction requiring dialysis |
| Duration of symptoms | 1 year | 1.2 years |
| Hb (g/dL) | 6.2 | 8.3 |
| TLC (×106/µL) | 5.2 | 7.3 |
| Platelets (×106/µL) | 246 | 187 |
| Peripheral blood smear | Normocytic normochromic anemia, mild rouleaux formation | Normocytic normochromic anemia |
| Creatinine (mg/dL) | 4.5 | 10.18 |
| 24-h urine protein (g/24 h) | 1.5 | 1.8 |
| SPEP (M-spike) (g/dL)/IFE | 0.8, IgG kappa | 0.2, IgG lambda |
| sFLC (kappa: lambda) | 4.9 | 0.2 |
| Imaging (whole-body CT scan/skeletal survey) | No skeletal lesions | No skeletal lesions |
| Renal biopsy (MGRS-related lesion) | C3 glomerulopathy | MIDD |
| Plasma cell % on bone marrow aspirate and biopsy | 6%; 15% binucleate forms and Dutcher bodies seen | 9%; 9% |
| IHC | Polyclonal pattern | Polyclonal pattern |
| Flow cytometry % Abnormal plasma cells in viable nucleated cells Abnormal to total plasma cell ratio | ||
| 0.1% 0.5 | 0.8% 0.8 | |
| FISH panel for del 13q14.3, del 17p13, t (4;14), t (11;4), t (14;16) | Inadequate sample | del 13q14.3 was found in 13% of plasma cells |
| Therapy | Received three cycles of VCD | Completed two cycles of VCD |
| Response | Normal serum free light chain ratio, M spike- 0.35 g/dL, Hb- 8 g/dL with infrequent transfusion requirement | Reduced frequency of dialysis from twice a month before diagnosis to once in last 2 months post initiation of therapy |
CT=computed tomography, FISH=fluorescence in situ hybridization, Hb=hemoglobin, IHC=immunohistochemistry, MGRS=monoclonal gammopathy of renal significance, VCD=bortezomib, dexamethasone, and cyclophosphamide, SPEP=serum protein electrophoresis, IFE=Immunofixation electrophoresis, sFLC=serum free light chain, MIDD=Monoclonal immune deposit disease
FCM for PCs was performed on the bone marrow (BM) sample collected in ethylenediaminetetraacetic acid (EDTA). The sample was lysed and staining done using a panel of antibodies against CD38-APC-Cy7, CD138-PE, CD45-PerCP-Cy5.5, CD19-PE-Cy7, CD27-FITC, CD81-FITC, CD56-APC, CD117-APC, intracellular anti-kappa-APC, and anti-lambda-FITC. Specimens were acquired using three-laser BD FACS Canto-II (BD Biosciences, San Jose, CA, USA) and analyzed on BD FACS Diva software version 8.0.1.
Case 1 showed 0.2% PCs on CD38, CD138, CD45, and side scatter (SSC) gating. Of these, half, that is, 0.1% PCs, showed an abnormal immunophenotype (CD56+/CD19−/CD81−/CD45) with a κ-restriction [Figure 1]. Case 2 showed 0.9% PCs on CD38, CD138, CD45, and SSC gating, including 0.8% λ-clonal PCs with an abnormal immunophenotype (CD56+/CD19−/CD27−/CD45 partial loss).
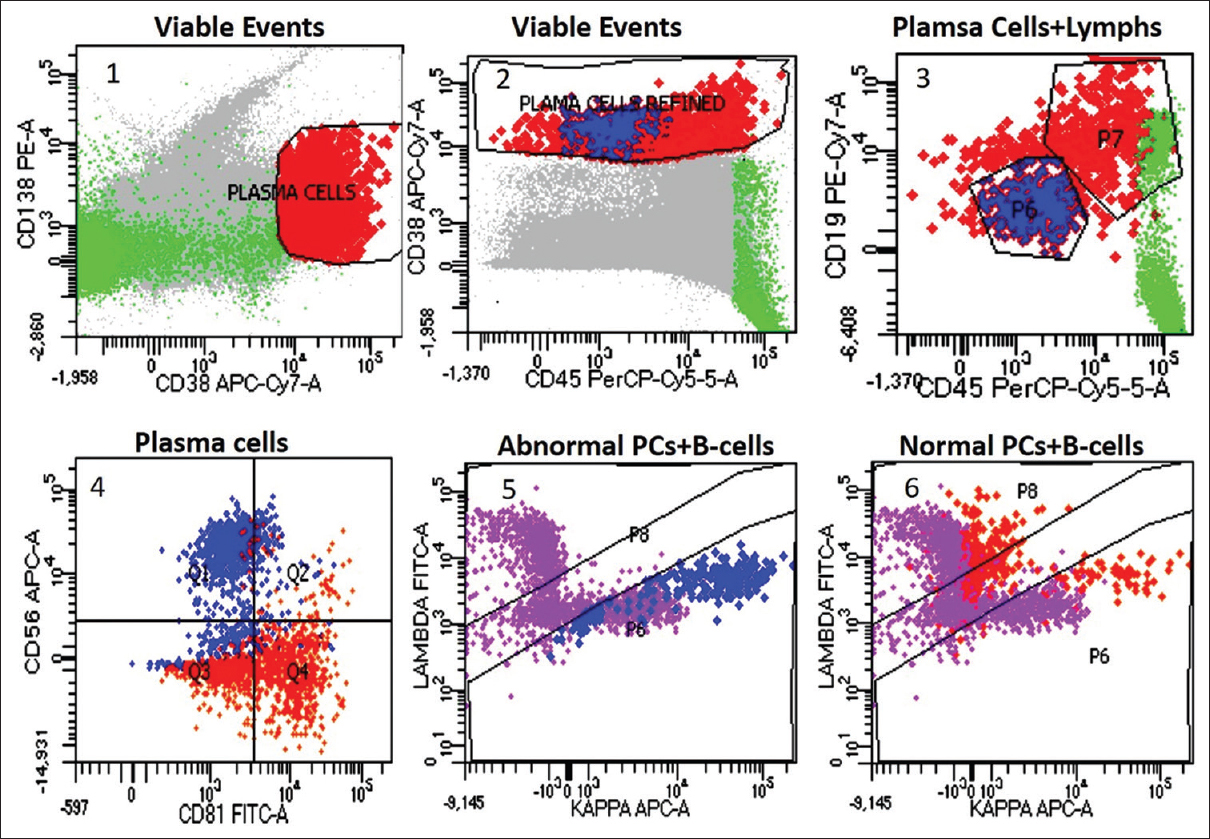
- Plasma cell immunophenotyping in case 1: plasma cell gating on CD38/CD138 (plot 1), plasma cell gate refinement on CD38/CD45 (plot 2), abnormal PCs (blue), normal PCs (orange), and mature lymphocytes (green) on CD19/CD45 (plot 3), CD56 expression with CD81 loss in abnormal PCs, while the normal PCs express CD81 and are negative for CD56 (plot 4), κ-restricted abnormal PCs and polyclonal B cells (purple) (plot 5), polyclonal normal PCs (orange) and B cells (purple) (plot 6). PC = plasma cell
In both cases, clonality in BM could be proven on FCM, whereas immunohistochemistry showed a polyclonal population. Clonal identification is important as same renal lesions can be found in different hematological disorders and treatment varies depending upon the type of clone (B cell/PC).[2] Immunohistochemistry could be useful only when a major PC clone is present and polyclonal population is lacking.[2] However, immunohistochemistry has low sensitivity when less number of abnormal PCs are admixed with polyclonal population.[3] FCM has the advantage of studying a large number of cells and simultaneous measurement of multiple antigenic expressions. Sensitive FCM can detect monoclonal PCs at a sensitivity of 10 − 4–10 − 6 and can discriminate between MGUS and MM. The number of residual polyclonal PCs is a useful discriminating marker between MGUS and MM.[5] MGUS usually has more than 5% normal plasma cells (NPCs) within total BM PCs (both our cases showed NPCs of 50% and 20%, respectively).[5]
To conclude, characterization and clonality identification of PCs or B cells in BM by FCM is a must in cases of MGRS as it is highly sensitive and guides in appropriate decision-making to guide correct therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge the staff of Department of Hematology, AIIMS, New Delhi.
References
- The evaluation of monoclonal gammopathy of renal significance:A consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019;15:45-59.
- [Google Scholar]
- Pathophysiology and management of monoclonal gammopathy of renal significance. Blood Adv. 2019;3:2409-23.
- [Google Scholar]
- Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol. 2010;23:433-51.
- [Google Scholar]
- New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586-92.
- [Google Scholar]






