Translate this page into:
Focal Crescentic Glomerulonephritis Superimposed on Myeloproliferative Disease-Related Glomerulopathy in a Case of Myelofibrosis
Corresponding Author: Dr. Janmejay Ashvinkumar Kunpara, “Vatsalya”, Shakti Plot 8 Corner, Sardarnagar, Morbi, 363641, Gujarat, India. E-mail: jak_924@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Kunpara JA, Darji BP, Patel HA, Patwari D, Patel SS, Darji PI. Focal Crescentic Glomerulonephritis Superimposed on Myeloproliferative Disease-Related Glomerulopathy in a Case of Myelofibrosis. Indian J Nephrol 2024;34:64-6. doi: 10.4103/ijn.ijn_218_22
Abstract
Proliferative glomerulonephritis in myelofibrosis is a very rare. Mesangial proliferation and sclerosis with changes of chronic thrombotic microangiopathy have been reported, but pauci-immune focal crescentic glomerulonephritis has not been described so far. Herein, we present a 68-year-old male who was a known case of myelofibrosis and presented with rapidly progressive glomerulonephritis and nephrotic range proteinuria. He was diagnosed as anti-neutrophil cytoplasmic antibody (ANCA)-negative focal crescentic glomerulonephritis, and he responded well to a course of intravenous methylprednisolone and cyclophosphamide. Pauci-immune focal crescentic glomerulonephritis may occur in myelofibrosis without ANCA and may be related to unknown pathogenetic mechanisms in myeloproliferative disorders or suggest any superimposed pathology that might respond well to immunosuppressants.
Keywords
Glomerulonephritis
myelofibrosis
pauci-immune
proliferative
Introduction
Myeloproliferative neoplasms are a group of clonal hematopoietic stem cell disorders characterized by proliferation of one or more myeloid lineages. Renal manifestations in myeloproliferative neoplasms mainly include hyperuricemia1 and renal vein thrombosis,2,3 but specific glomerular lesions are a rare. Few studies have found Focal segmental glomerulosclerosis (FSGS) and mesangial sclerosis with myeloproliferative disorder.4 In 2011, Said et al.5 described the term myeloproliferative disorder-related glomerulopathy (MDRG). Other lesions such as extramedullary hematopoiesis have also been described in primary myelofibrosis.6 Literature has not described anti-neutrophil cytoplasmic antibody (ANCA)-negative pauci-immune proliferative glomerulonephritis in this group of patients.
Case Report
A 68-year-old male presented with complaints of nocturnal frequency of urine (three to four times/night) and generalized weakness in the last 1 week. He did not have decreased appetite, nausea, vomiting, itching, or any lower urinary tract complaints. He also did not complain of edema, red-colored urine, joint pain, recent throat pain, skin rashes, or frothing of urine. He also denied taking any nonsteroidal anti-inflammatory drugs, aminoglycosides, or any other nephrotoxic drugs. He had a history of hypertension for 20 years and biopsy-proven myelofibrosis [Figure 1] for 10 years with JAK2 mutation positivity, for which he was on hydroxyurea and aspirin. On examination, his blood pressure was 140/70 mmHg with mild pallor and splenomegaly. His a hemoglobin (Hb) was 9.7 mg/dl, platelet count 4.15 lakhs, and S. creatinine 2.6 mg/dl. His urine examination revealed proteinuria of 4.4 g/day with seven white blood cells and 4-6 dysmorphic RBCs/High power field (HPF), with a normal serum albumin of 4 mg/dl and serum cholesterol 180 mg/dl. His random blood sugar was 210 mg/dl and hemoglobin A1c (HbA1c) was 6.5%. His creatinine rapidly rose to 4.6 mg/dl in a week. Complement C3 (C3) level was normal, ANCA by immunofluorescence was negative, and there was no monoclonal band on serum protein electrophoresis. His ultrasonography showed normal to mildly enlarged kidneys with normal echogenicity and corticomedullary differentiation with normal renal arterial and venous Doppler. Kidney biopsy which showed 3/10 glomeruli globally sclerosed, with a partial cellular crescent [Figure 2] in one glomerulus and rest of the glomeruli showing mild mesangial proliferation with normal glomerular basement membrane and normal capillary lumens without any fibrin thrombi, mild mixed infiltration in the interstitium, and mild interstitial edema and occasional foci of tubular atrophy with no interstitial fibrosis and normal blood vessels. No immune deposits were found in immunofluorescence study, and Congo red stain was negative. On electron microscopy, there were four glomeruli, of which two were globally sclerosed and the others showed focal foot process effacement and normal basement membrane. There was focal deposition of mesangial and subendothelial electron-dense deposits [Figure 3] and few foci of tubuloreticular lesions in endothelial cells without any subendothelial electrolucent fluffy deposit and normal capillary lumen [Figure 4]. Since immunofluorescence showed no immune deposits and electron microscopy showed subendothelial electron-dense deposits on the background of a long history of myelofibrosis, we believe this may be a focal crescentic glomerulonephritis superimposed on myelofibrosis-related glomerulopathy.
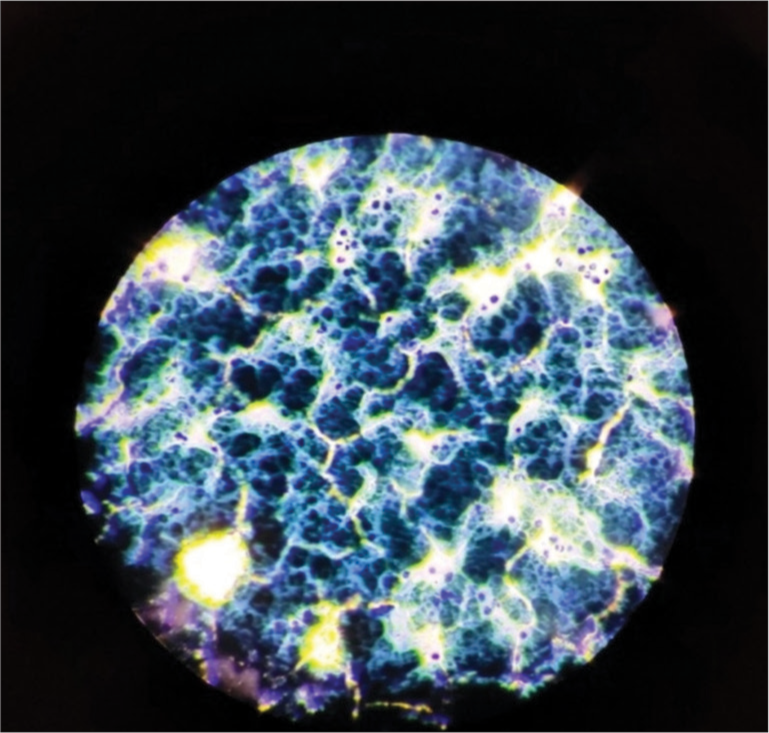
- Bone marrow biopsy showing changes in myelofibrosis (40 × magnification).

- A glomerulus showing proliferative changes with partial cellular crescent under light microscopy (hematoxylin and eosin stain, ×40).
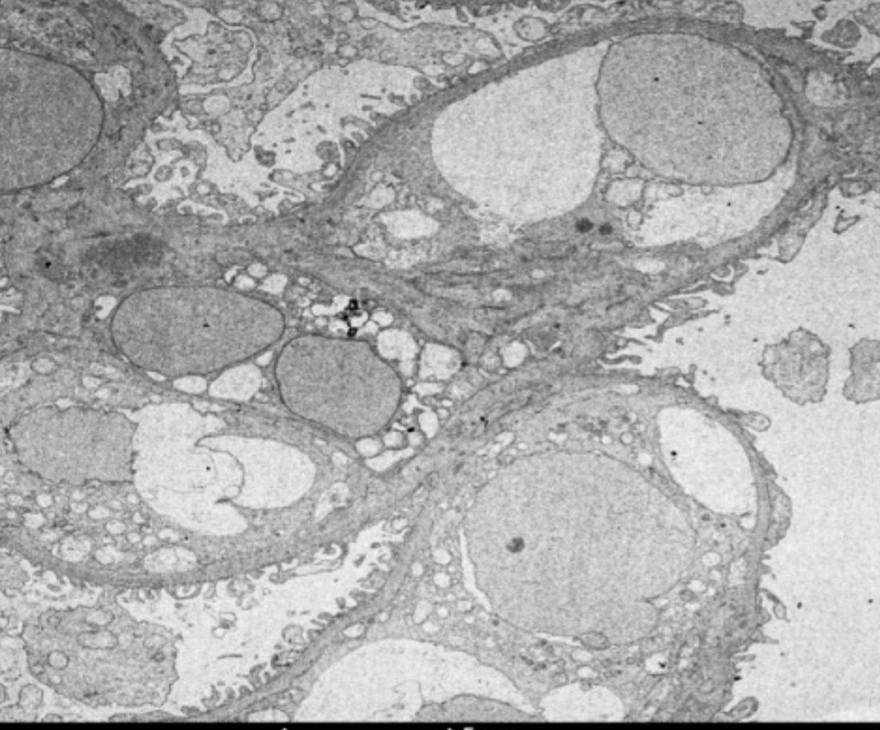
- Electron microscopy showing focal effacement of foot process and few mesangial and subendothelial deposits (magnification: 2000×).
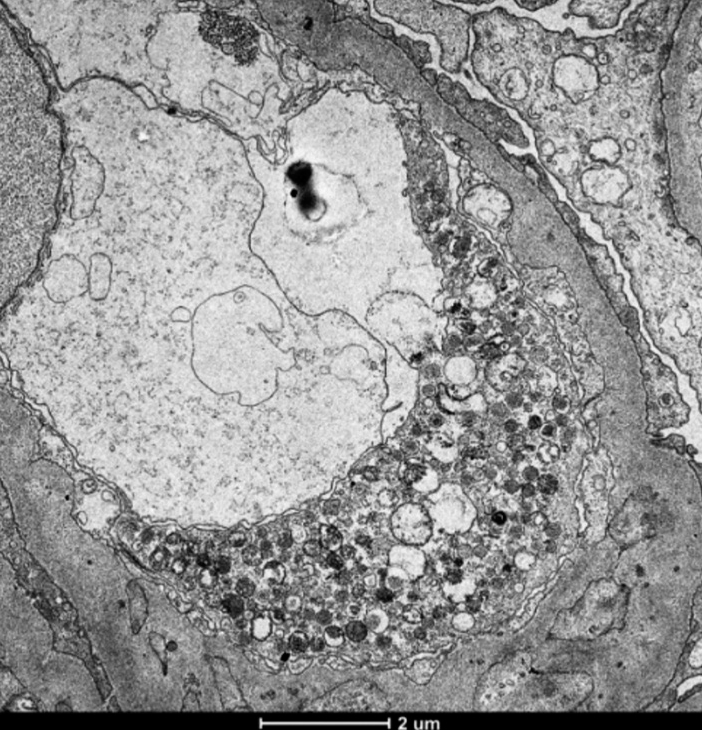
- Electron microscopy showing tubuloreticular inclusions in endothelial cells (magnification: 5300×).
We started with pulse methylprednisolone for 3 days followed by oral prednisolone 1 mg/kg per day and intravenous (IV) cyclophosphamide every 15th day. He improved in terms of symptoms; also, his serum creatinine came down to 1.5 mg/dl after four doses of cyclophosphamide. Oral prednisolone was tapered to 10 mg/day in 8 weeks.
Discussion
Myeloproliferative disorder is a clonal hematopoietic stem cell disorder with proliferation of one or more myeloid lineages.7 Primary myelofibrosis, a type of myeloproliferative disorder, is characterized by predominant proliferation of megakaryocytes and granulocytes in bone marrow, eventually leading to fibrosis.8 Major complications of primary myelofibrosis are hemorrhage, thrombosis, and transformation into acute myeloid leukemia.9 Renal involvement in primary myelofibrosis is generally secondary to hyperuricemia and renal vein thrombosis, with rare involvement of the glomerular compartment with mesangial proliferation, which is usually pauci-immune on IF. According to the study by Said et al.,5 patients with primary myelofibrosis have a higher risk of developing myeloproliferative disorder-related glomerulopathy. In this case, there was myelofibrosis of long duration and renal dysfunction with nephrotic range proteinuria and normal serum complement level. Biopsy showed a partial cellular crescent in one glomerulus, with other glomeruli showing sclerosis, mesangial proliferation, and negative IF, with presence of focal foot process effacement in electron microscopy, but there were no signs of chronic TMA such as glomerular double contouring, arterial thrombosis, intracapillary fibrin thrombi, or any subendothelial electrolucent fluffy material, and the interstitium showed mixed inflammatory cell infiltration. Also, any megakaryocyte or immature myeloid cell was not present in capillaries. This patient also presented late in the course of myelofibrosis, which is consistent with other reports in the literature.
The molecular pathogenesis of these findings on biopsy has not been elucidated. It has been postulated that high levels of platelet-derived growth factor (PDGF) and transforming growth factor (TGF)-β derived from megakaryocytes are associated with primary myelofibrosis and are responsible for its progression to fibrosis.10,11 Studies have shown that PDGF and TGF-β stimulate mesangial cell proliferation, mesangial matrix synthesis, apoptosis of podocytes, and secretion of fibronectin and collagen from mesangial cells.12,13
Previously, a case of profibrotic myelofibrosis with crescentic glomerulonephritis and immune complex deposition has been described,14 but pauci-immune mesangioproliferative glomerulonephritis with crescent in myelofibrosis is atypical and should alert one to the presence of an alternate pathology which may respond to immunosuppressant.
Conclusion
Myeloproliferative disorders can present with renal involvement in various ways including myeloproliferative disorder-related glomerulopathy. It occurs late in the course of disease and may be related to some unknown pathogenetic mechanism. The common histologic features include mesangial expansion and sclerosis, features of chronic thrombotic microangiopathy, absence of immune deposits, and poor response to steroid therapy. This case suggests that other pathologies such as pauci-immune focal necrotizing glomerulonephritis may be superimposed on the histologic findings of myeloproliferative disorder-related glomerulopathy and may be more amenable to treatment with immunosuppression.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Physiology of hyperuricemia and urate-lowering treatments. Front Med (Lausanne). 2018;5:160.
- [CrossRef] [PubMed] [Google Scholar]
- Acute renal infarction: A presentation of essential thrombocytosis. Kidney Int. 2017;92:1292.
- [CrossRef] [PubMed] [Google Scholar]
- Leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera: A systematic review and meta-analysis. Blood Adv. 2019;3:1729-37.
- [CrossRef] [PubMed] [Google Scholar]
- Focal segmental glomerulosclerosis and mesangial sclerosis associated with myeloproliferative disorders. Am J Kidney Dis. 1999;34:889-93.
- [CrossRef] [PubMed] [Google Scholar]
- Myeloproliferative neoplasms cause glomerulopathy. Kidney Int. 2011;80:753-9.
- [CrossRef] [PubMed] [Google Scholar]
- Renal disease associated with myeloproliferative neoplasms and myelodysplastic syndrome/myeloproliferative neoplasms. Histopathology. 2021;78:738-48.
- [CrossRef] [PubMed] [Google Scholar]
- The myeloproliferative disorders. N Engl J Med. 2006;355:2452-66.
- [CrossRef] [PubMed] [Google Scholar]
- Classification and diagnosis of myeloproliferative neoplasms. The 2008 world health organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14-22.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombosis and hemorrhage in polycythemia vera and essential thrombocytopenia. Br J Haematol. 2005;128:275-90.
- [CrossRef] [PubMed] [Google Scholar]
- TGF-beta and megakaryocytes in the pathogenesis of myelofibrosis in myeloproliferative disorders. Leuk Lymphoma. 1995;20:39-44.
- [CrossRef] [PubMed] [Google Scholar]
- Increased intraplatelet levels of platelet-derived growth factor and transforming growth factor-beta in patients with myelofibrosis with myeloid metaplasia. Br J Haematol. 1991;77:80-6.
- [CrossRef] [Google Scholar]
- A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19:12-23.
- [CrossRef] [PubMed] [Google Scholar]
- Aldose reductase regulates TGF-beta1induced production of fibronectin and type IV collagen in cultured rat mesangial cells. Nephrology (Carlton). 2006;11:105-12.
- [CrossRef] [PubMed] [Google Scholar]
- Immune complex associated glomerulonephritis in a patient with prefibrotic primary myelofibrosis: A case report. Indian J Nephrol. 2021;31:50-3.
- [CrossRef] [PubMed] [Google Scholar]







