Translate this page into:
Gender Disparity in Hemodialysis Practices and Mortality: A Nationwide Cross-Sectional Observational Study
Corresponding author: Mythri Shankar, Department of Nephrology, Institute of Nephro-Urology, Bangalore, Karnataka, India. E-mail: mythri.nish@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shankar M, Sankarasubaiyan S, Kasiviswanathan S, Shah KD, Luyckx V. Gender Disparity in Hemodialysis Practices and Mortality: A Nationwide Cross-Sectional Observational Study. Indian J Nephrol. 2024;34:609-16. doi: 10.25259/ijn_559_23
Abstract
Background
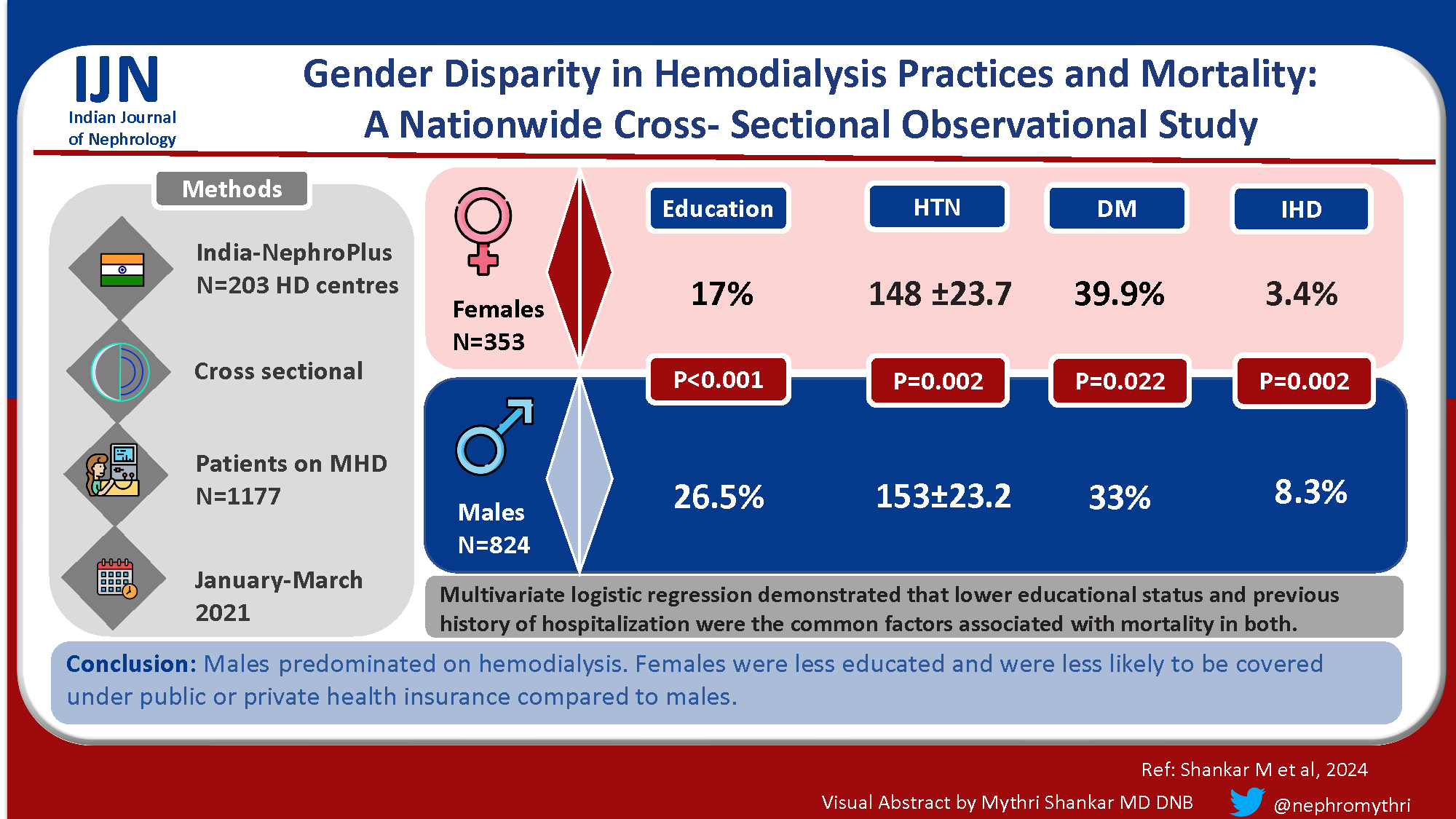
Disparities between genders are well documented in incidence, progression, and outcomes of chronic kidney disease (CKD). This study aimed to describe demographic characteristics, clinical and socio-economic factors among males and females on maintenance hemodialysis and to determine any association with mortality among males and females.
Materials and Methods
A nationwide cross-sectional study was conducted in a hemodialysis network in India. All adult (>18 years) patients who died while receiving maintenance hemodialysis and an equal number of surviving control patients on maintenance hemodialysis (MHD) between January 1, 2021 to March 31, 2021 were included in the study. The demographic, socioeconomic, and hemodialysis factors were compared between both the genders.
Results
A total of 1177 patients who died during the study period were included. The majority were males (824, 70.01%). Males were more educated than females (P < 0.001). The proportion of female patients dialysed with temporary catheters where more than males, who had definite vascular access such as AV fistula or AV graft (P < 0.001). More female patients required out-of-pocket expenditure (P = 0.005). Multivariate logistic regression demonstrated that lower educational status, hypoalbuminemia, previous history of hospitalization, and dialysis in centres run by Public Private Partnership (PPP) were associated with mortality in males. Lower educational status, heart failure and previous history of hospitalization were the factors associated with mortality in females.
Conclusion
Males predominated on hemodialysis. Females were less educated and were less likely to be covered under public or private health insurance compared to males.
Keywords
Gender
Hemodialysis
Mortality
Male
Female
Introduction
In the general population,1 women tend to have higher survival rates than men, which may be attributable to their lower occurrence of cardiovascular risk factors and diseases.2,3 Recognized physiological differences between genders may contribute to reported disparities in various diseases, including type 2 diabetes, cardiovascular disease, depression, and various stages of kidney disease.4-13 The progression rate of numerous kidney disorders is influenced by gender, a topic that has been extensively examined in more comprehensive reviews.14 The largest meta-analysis conducted to date, encompassing over 11,000 patients from 68 different studies, has shown that women with conditions such as polycystic kidney disease, IgA nephropathy, membranous nephropathy, and ‘chronic kidney disease of unknown etiology’ tend to experience a slower progression of kidney disease compared to men with matching blood pressure and lipid levels who have these conditions.15 More recently, two additional population-based studies have also revealed that men exhibit a slower chronic kidney disease (CKD) progression compared to women.16,17 Previous observational studies from the Dialysis Outcomes and Practice Patterns Study (DOPPS)18 and the Austrian Dialysis Registry,19 have shown no difference in survival between men and women. The present study aims to perform gender-specific analysis of maintenance hemodialysis (MHD) practices and mortality.
Materials and Methods
A nationwide cross-sectional study was conducted in India among patients undergoing MHD in 203 centers provided by a single large network-Nephroplus. There were a total of 9 (4.43%) standalone centers and 194 (95.57%) were part of an institution/hospital. Region-wise distribution of centers is provided in Table 1. Data was collected retrospectively from the hospital management information system and electronic medical records. All adult (>18 years) patients receiving MHD from January 1, 2021 to March 31 2021 were included. Patients who died were identified and an equal number of survivors were randomly selected from the total dialysis population in the dialysis network. We excluded patients on HD for less than 90 days, aged less than 18 years, and deaths due to COVID-19 infection. The patients who died during this period were considered as cases and those who survived were taken as controls [Figure 1]. We stratified the non-survivors (cases) and survivors (controls) by gender to identify gender-specific risk factors for death.
| Region | Number of centers | Percentage |
|---|---|---|
| East | 49 | 24.14 |
| North | 40 | 19.7 |
| South | 78 | 38.421 |
| West | 36 | 17.73 |
| Grand total | 203 | 100% |
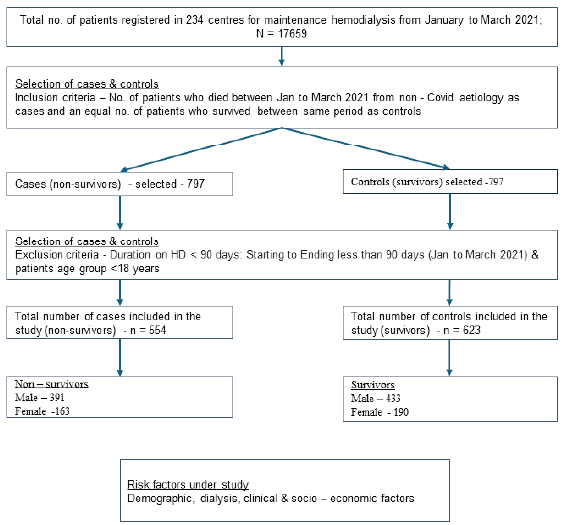
- Methodology for selection of cases and controls for the study.
Data on demographic factors, dialysis, educational status, and dialysis payer type (public insurance, out-of-pocket payment, or private insurance) were collected. HD facilities were classified by funding model as public–private partnerships (PPP) or private. PPP is a setup where the government pays for HD in a private dialysis center. Dialysis-related factors included dialysis vintage, frequency of weekly HD sessions, vascular access type, dialysis adequacy (measured online in Fresenius 4008 S machines), hemoglobin, erythropoietin use, and serum albumin levels. Additionally, the cause of CKD (diabetic or non-diabetic), and comorbidities including hypertension, history of heart failure, and any hospitalizations in the past 3 months were also documented. Ethical approval for this study was waived by the Institutional Ethics Committee of The George Institute for Global Health India. This is due to our exclusive use of deidentified data from patients who had voluntarily consented to its use for clinical research at the time of registration at a NephroPlus centre, before any data was collected. A refusal to consent did not impact a patient’s right to receive treatment, which was made clear to patients from the outset.
Statistical analysis
We report all continuous variables as mean ± standard deviation (SD). The differences between groups were tested using an independent sample t-test. Other variables are presented as numbers (n) and proportions (%). Following this, both univariate and multivariable logistic regression analyses were conducted to ascertain the death risk in terms of odds ratios, accompanied by a 95% confidence interval. All variables were subjected to univariate analysis and for the multivariable logistic analysis, all significant risk factors (P < 0.05) were included, applying a backward elimination approach to determine which factors to include in the model.
We analyzed the data using STATA software version 17.0. (Ref: StataCorp. STATA statistical software: Release 17. College section, TX; StataCorp LLC) The significance level was fixed at 5% (P value <0.05) with 95% confidence interval (CI).20–24
Among patients dialyzed across 203 centers, 554 patients who died from non-COVID etiologies were included as cases and 623 survivors as controls during the study period between January 1, 2021 and March 31, 2021 and were stratified based on gender. Males were 824 (70.01%) and 353 (29.99%) were females. All patients had received maintenance HD for a minimum period of 90 days. The selection of patients is illustrated in Figure 1. The comparison of demographic, HD, clinical, and socioeconomic characteristics between men and women is shown in Table 2.
| Characteristics | Males (N = 824) | Females (N = 353) | P-value | ||
|---|---|---|---|---|---|
| Demographic factors | |||||
| Age (years) | 55.16 ± 13.2 | 55.14 ± 13.3 | 0.981 | ||
| Education | |||||
| Illiterate | 215 (26.1%) | 136 (38.5%) |
0.00 |
||
| High school | 230 (27.9%) | 113 (32.0%) | |||
| Higher secondary | 161 (19.5%) | 44 (12.5%) | |||
| College | 218 (26.5%) | 60 (17.0%) | |||
| BMI (Indian) | 22.55 ± 4.2 | 22.82 ± 5.5 | 0.364 | ||
| Zone | |||||
| North | 173 (21.0%) | 94 (26.6%) |
0.092 |
||
| East | 122 (14.8%) | 45 (12.7%) | |||
| West | 126 (15.3%) | 61 (17.3%) | |||
| South | 403 (48.9%) | 153 (43.3%) | |||
| Dialysis factors | |||||
| HD duration (months) | 22.72 ± 17.0 | 24.75 ± 17.8 | 0.065 | ||
| HD frequency (per week) | |||||
| 1X | 15 (1.8%) | 8 (2.3%) |
0.044 |
||
| 2X | 434 (52.7%) | 186 (52.7%) | |||
| 3X | 366 (44.4%) | 156 (44.2%) | |||
| Irregular/SOS | 9 (1.1%) | 3 (0.8%) | |||
| Vascular access | |||||
| AVF/AVG | 718 (87.1%) | 278 (78.8%) |
<0.001 |
||
| Catheter | 106 (12.9%) | 75 (21.2%) | |||
| Dialysis adequacy | 1.26 ± 0.1 | 1.25 ± 0.1 | 0.338 | ||
| Clinical factors | |||||
| Hb level g/dl | 9.12 ± 1.6 | 8.95 ± 1.4 | 0.090 | ||
| Serum albumin g/dl | 3.53 ± 0.5 | 3.54 ± 0.5 | 0.860 | ||
| EPO use | |||||
| Yes | 786 (95.4%) | 342 (96.9%) |
0.239 |
||
| No | 38 (4.6%) | 11 (3.1%) | |||
| Cause of CKD | |||||
| No diabetes | 552 (67.0%) | 212 (60.1%) | 0.022 | ||
| Diabetes | 272 (33.0%) | 141 (39.9%) | |||
| HTN | |||||
| Mean elevated systolic blood pressure (>/=140 mmHg) | 153 ± 23.2 | 148 ± 23.7 | 0.002 | ||
| Mean elevated diastolic blood pressure (>/=90 mmHg) | 83 ± 11.5 | 81 ± 11.9 | 0.027 | ||
| History of heart failure |
0.068 |
||||
| Yes | 61 (7.4%) | 16 (4.5%) | |||
| No | 763 (92.6%) | 337 (95.5%) | |||
| History of ischemic heart disease | |||||
| Yes | 68 (8.3%) | 12 (3.4%) | |||
| No | 756 (91.7%) | 341 (96.6%) | 0.002 | ||
| Hospitalization in previous 3 months | |||||
| Yes | 186 (22.6%) | 87 (24.6%) | 0.440 | ||
| No | 638 (77.4%) | 266 (75.4%) | |||
| Outcome | |||||
| Survivors | 433 (52.5%) | 190 (53.8%) | 0.688 | ||
| Non-survivors | 391 (47.5%) | 163 (46.2%) | |||
| Socio-economic factors | |||||
| Payer type | |||||
| Out-of-pocket | 224 (27.2%) | 121 (34.3%) | 0.013 | ||
| Private insurance | 257 (31.2%) | 104 (29.5%) | |||
| Public insurance | 337 (40.9%) | 121 (34.3%) | |||
| Mixed | 6 (0.7%) | 7 (2.0%) | |||
| Type of center | |||||
| Public–private | 371 (45.0%) | 128 (36.3%) | 0.005 | ||
| Private hospital-based | 453 (55.0%) | 225 (63.7%) | |||
P value is considered significant if < 0.05. The values in bold are significant P values. BMI: Body mass index; HD: Hemodialysis; AVF/AVG: Arterio-venous fistula/Arterio-venous graft; HTN: Hypertension; CKD: Chronic kidney disease; Hb: Hemoglobin; EPO: Erythropoietin.
The mean age was 55.16 ± 13.2 years in males and 55.14 ± 13.3 years in females. Females received less education (P = <0.001). More females were dialyzed with temporary catheters compared to AV fistulas at the time of initiation of HD (P < 0.001). Requirement of out-of-pocket expenditure was more common among females (P = 0.005). Hypertension and ischemic heart disease (IHD) were significantly more prevalent in males (P = 0.02), while diabetes as the cause of kidney failure was more prevalent in females (P = 0.022). However, there was no difference in body mass index (BMI) (P = 0.36), dialysis adequacy (P = 0.33), EPO use (P = 0.239), or hospitalization rates (P = 0.44) between the groups.
Among 824 males, 391 were non-survivors and 433 were survivors. Among 353 females, 163 were non-survivors and 190 were survivors. Table 3 shows the comparison between non-survivors and survivors of both genders. Among males, lower educational status, lower BMI, use of temporary HD catheters for initiation of dialysis, anemia, hypoalbuminemia, diabetes, hypertension, history of IHD, history of previous hospitalization, out-of-pocket expenditure and receiving dialysis in centers run through PPPs were significantly associated with death. Among females, lower educational status, anemia, hypoalbuminemia, history of IHD, history of previous hospitalization, out-of-pocket expenditure and receiving dialysis in centers run by PPPs were significantly associated with death [Table 3].
| Variables | Males (N = 824) | P-value | Females (N = 353) | P-value | ||
|---|---|---|---|---|---|---|
| S (433) | NS (391) | S (190) | NS (163) | |||
| Demographic factors | ||||||
| Age (years) | 56.13 ± 12.6 | 54.52 ± 13.7 | 0.080 | 55.51 ± 12.9 | 53.63 ± 14.21 | 0.194 |
| Education | <0.001 | 0.000 | ||||
| Illiterate | 105 (24.2) | 110 (28.1) | 49 (25.8) | 87 (53.4) | ||
| High school | 94 (21.7) | 136 (34.8) | 78 (41.1) | 35 (21.5) | ||
| Higher secondary | 911 (21) | 70 (17.9) | 25 (13.2) | 19 (11.7) | ||
| College | 43 (33) | 75 (19.2) | 38 (20) | 22 (13.5) | ||
| BMI (Indian) | 22.98 ± 4.1 | 22.07 ± 4.3 | 0.002 | 22.65 ± 5.5 | 23.01 ± 5.5 | 0.537 |
| Zone | ||||||
| North | 117 (27) | 56 (14.3) | <0.001 | 54 (28.4) | 40 (24.5) | 0.030 |
| East | 58 (13.4) | 64 (16.4) | 25 (13.2) | 20 (12.3) | ||
| West | 77 (17.84) | 49 (12.5) | 41 (21.6) | 20 (12.3) | ||
| South | 181 (1.8) | 222 (56.8) | 70 (36.8) | 83 (50.9) | ||
| Dialysis factors | ||||||
| HD duration (years) | 23.91 ± 17.8 | 21.4 ± 16 | 0.035 | 26.56 ± 18.7 | 22.64 ± 16.4 | 0.039 |
| HD frequency | 0.486 | |||||
| 1X | 6 (1.4) | 9 (2.3) | 0.587 | 3 (1.6) | 5 (3.1) | |
| 2X | 232 (53.6) | 202 (51.7) | 106 (55.8) | 80 (49.1) | ||
| 3X | 189 (43.6) | 177 (45.3) | 79 (41.6) | 77 (47.2) | ||
| Irregular/SOS | 6 (1.4) | 3 (0.8) | 2 (1.1) | 1 (0.6) | ||
| Vascular access | ||||||
| AVF/AVG | 389 (89.8) | 329 (84.1) | 0.015 | 154 (81.1) | 124 (76.1) | 0.254 |
| Catheter | 44 (10.2) | 62 (15.9) | 36 (18.9) | 39 (23.9) | ||
| Dialysis adequacy (Kt/V) | 1.05 ± 0.2 | 1.08 ± 0.2 | 0.073 | 1.06 ± 0.2 | 1.08 ± 0.2 | 0.527 |
| Clinical factors | ||||||
| Hemoglobin (g/dl) | 9.38 ± 1.5 | 8.83 ± 1.6 | <0.001 | 9.22 ± 1.4 | 8.62 ± 1.3 | <0.001 |
| Serum albumin (g/dl) | 3.32 ± 0.4 | 1.57 ± 0.4 | <0.001 | 3.33 ± 0.4 | 1.51 ± 0.5 | 0.003 |
| EPO use | ||||||
| Yes | 409 (94.5) | 377 (96.4) | 0.180 | 183 (96.3) | 159 (97.5) | 0.507 |
| No | 24 (5.5) | 14 (3.6) | 7 (3.7) | 4 (2.5) | ||
| Cause of CKD | ||||||
| Non-diabetic | 304 (70.2) | 248 (63.4) | 0.039 | 117 (61.6) | 95 (58.3) | 0.528 |
| Diabetic | 129 (29.8) | 143 (36.6) | 73 (38.4) | 68 (41.7) | ||
| HTN | ||||||
| Systolic blood pressure (>/=140 mmHg) | 151 ± 20.5 | 155 ± 25.7 | 0.015 | 147 ± 20 | 149 ± 27.4 | 0.401 |
| Diastolic blood pressure (>/=90 mmHg) | 83 ± 10.7 | 83 ± 12.2 | 0.979 | 81 ± 11.6 | 82 ± 12.1 | 0.372 |
| History of heart failure | ||||||
| Yes | 29 (6.7) | 32 (8.2) | 0.416 | 3 (1.6) | 13 (8) | 0.004 |
| No | 404 (93.3) | 359 (91.8) | 187 (98.4) | 150 (92) | ||
| History of ischemic heart disease | ||||||
| Yes | 23 (5.3) | 45 (11.5) | 0.001 | 2 (1.1) | 10 (6.1) | 0.009 |
| No | 410 (94.7) | 346 (88.5) | 188 (98.9) | 153 (93.9) | ||
| Hospitalization in previous 3 months | ||||||
| Yes | 43 (9.9) | 143 (36.6) | <0.001 | 28 (14.7) | 59 (36.2) | <0.001 |
| No | 390 (90.1) | 248 (63.4) | 162 (85.3) | 104 (63.8) | ||
| Socioeconomic factors | ||||||
| Payer type | ||||||
| Out of pocket | 120 (27.7) | 104 (26.6) | <0.001 | 75 (39.5) | 46 (28.2) | <0.001 |
| Private insurance | 174 (40.2) | 83 (21.2) | 69 (36.3) | 35 (21.5) | ||
| Public insurance | 139 (32.1) | 198 (50.6) | 46 (24.2) | 75 (46) | ||
| Mixed | 0 | 6 (1.5) | 0 | 7 (4.3) | ||
| Type of center | ||||||
| Public–private partnership | 165 (38.1) | 206 (52.7) | <0.001 | 51 (26.8) | 77 (47.2) | <0.001 |
| Private hospital-based | 268 (61.9) | 185 (47.3) | 139 (73.2) | 86 (52.8) | ||
P value is considered significant if <0.05. The values in bold are significant P values. BMI: Body mass index; HD: Hemodialysis; AVF/AVG: Arterio-venous fistula/Arterio-venous graft; HTN: Hypertension; CKD: Chronic kidney disease; Hb: Hemoglobin; EPO: Erythropoietin.
Univariate logistic regression analysis demonstrated that lower educational status, use of temporary HD catheters for initiation, lower BMI, lower dialysis adequacy, anemia, hypoalbuminemia, diabetes mellitus, history of hospitalization in the last 3 months, and HD centers run by PPP compared to only private HD centers were associated with mortality in males. Similarly, lower educational status, anemia, hypoalbuminemia, history of heart failure, hospitalization in the previous 3 months, HD centers run by PPPs were associated with mortality in females [Table 4]. Multivariate analysis has demonstrated that lower educational status, hypoalbuminemia, previous history of hospitalization (last 3 months), and patients from HD centers run by PPPs were the factors associated with mortality in males. Lower educational status, heart failure, and previous history of hospitalization were the factors associated with mortality in females [Table 5].
| Characteristics | Males Prevalence ratio (95% CI) | P-value | Females Prevalence ratio (95% CI) | P-value |
|---|---|---|---|---|
| Demographic factors | ||||
| Education status | ||||
| Illiterate | 1.487 (1.187, 1.862) | 0.001 | 1.744 (1.222,2.489) | 0.002 |
| High school | 1.718 (1.389,2.125) | <0.001 | 0.844 (0.548,1.300) | 0.444 |
| Higher secondary | 1.263 (0.980, 1.629) | 0.071 | 1.177 (0.732,1.893) | 0.500 |
| Dialysis factors | ||||
| Vascular access - Catheter | 1.276 (1.067,1.526) | 0.008 | 1.165 (0.904,1.502) | 0.236 |
| Dialysis adequacy < 1.2 | 1.262 (1.003,1.587) | 0.046 | 1.139 (0.777,1.670) | 0.504 |
| Clinical factors | ||||
| Hb level g/dl | ||||
| >12 | 1.020 (0.639,1.627) | 0.933 | – | – |
| 8–9.9 | 1.177 (0.969,1.429) | 0.099 | 1.442 (1.051,1.980) | 0.023 |
| <8 | 1.588 (1.302,1.938) | <0.001 | 1.808 (1.288,2.538) | 0.001 |
| Serum albumin g/dl <3.5 | 1.707 (1.430,2.038) | <0.001 | 1.494 (1.144,1.950) | 0.003 |
| Cause of CKD - diabetic | 1.170 (1.011,1.353) | 0.035 | 1.076 (0.857,1.350) | 0.526 |
| History of heart failure | 1.114 (0.867,1.432) | 0.395 | 1.825 (1.402,2.376) | <0.001 |
| History of ischemic heart disease | 1.445 (1.199,1.742) | <0.001 | 1.857 (1.405,2.455) | <0.001 |
| Hospitalization in previous 3 months | 1.977 (1.745,2.241) | <0.001 | 1.734 (1.408,2.136) | <0.001 |
| Socioeconomic factors | ||||
| Payer type | ||||
| Private insurance | 0.695 (0.554,0.872) | 0.002 | 0.885 (0.793,1.607) | 0.498 |
| Public insurance | 1.265 (1.071,1.495) | 0.006 | 1.630 (1.359,2.495) | <0.001 |
| Type of center - Public–private | 1.377 (1.191,1.591) | <0.001 | 0.616 (0.493,0.770) | <0.001 |
CKD: Chronic kidney disease, CI: confidence interval.
| Characteristics |
Males Adjusted prevalence ratio (95% CI) |
P-value |
Females Adjusted prevalence ratio (95% CI) |
P-value |
|---|---|---|---|---|
| Education status | ||||
| Illiterate | 1.386 (1.055, 1.819) | 0.019 | 1.909 (1.240, 2.939) | 0.003 |
| High school | 1.594 (1.259, 2.018) | <0.001 | 1.073 (0.704,1.633) | 0.742 |
| Higher secondary | 1.298 (0.987,1.706) | 0.062 | 1.287 (0.787, 2.104) | 0.313 |
| Clinical factors | ||||
| Serum albumin g/dl < 3.5 | 1.446 (1.223, 1.710) | <0.001 | 1.074 (0.819,1.408) | 0.605 |
| History of heart failure | 1.764 (1.199,2.595) | 0.004 | ||
| Hospitalization in previous months | 1.810 (1.541,2.124) | <0.001 | 2.056 (1.587, 2.663) | <0.001 |
| Socioeconomic factors | ||||
| Type of center | ||||
| Public–private | 1.387 (1.077, 1.787) | 0.011 | 1.282 (0.847,1.943) | 0.239 |
CI: confidence interval
Discussion
This is the first study from India, exploring factors associated with mortality among both the genders in patients undergoing maintenance hemodialysis. Earlier studies from dialysis cohorts25,26 showed that the life expectancy of women is not higher than men. While men generally show higher death rates than women in the overall population, DOPPS14 has shown equivalent mortality rates for both sexes, with the ratio near unity across all DOPPS countries, except Japan. Factors, including ethnic diversity, and disparities in healthcare access may influence the outcome.27–29 The greater impact of a higher BMI on the survival of male dialysis patients compared to female patients may be attributed to men having more skeletal muscle mass and less fat mass due to male sex hormones.30,31
Women had a significantly lower educational status than men and lower literacy level was associated with mortality in both genders. A systematic review of 29 studies showed that limited health literacy was found to be a significant and independent predictor of hospital admissions, emergency department visits, skipped dialysis treatments, cardiovascular incidents, and deaths.32 Yet, when literacy levels are compared between genders, a significant disparity is evident, with a noticeable difference in literacy rates between men and women in India.33,34
The requirement of out-of-pocket expenditure and use of temporary access for initiation of HD are significantly higher in females. The outcomes of this research align with those found in previous studies on healthcare expenditure (HCE) in India and certain Asian nations,34,35 but they diverge from findings in numerous developed and developing countries.36,37 Typically, women in developed countries possess greater health awareness, make more use of health services and preventive measures, and consequently spend more on their health care compared to those from developing countries.38 However, in nations like India and China, a tangled mix of poverty and societal stratification, often pushes women’s health down the list of household priorities, leading women to allocate more time to domestic or non-income-generating work.39 This could be the reason for women not being enrolled into state-sponsored or private insurance cover. Also, women may delay addressing their health needs to cater to the needs of the income-earning male members of the family and often give precedence to the health of males over their own40 which may potentially result in loss to follow-up, unable to buy medications, non-compliant to medications, failure to create AV fistula before progressing to end stage kidney disease and ending up with the requirement of temporary access for initiation of HD (urgent start HD). Therefore, the gender disparity in HCE in India might be due to the intersection of socioeconomic factors and patriarchal traditions.41
In this study, IHD was significantly more common in men than women. This is in concordance with previous data. Typically, women have a lower occurrence of IHD than men, except those who are 65 years old and above. As women reach menopause, the disparity in IHD onset between genders narrows, leading to a higher incidence in older women. The post-poned development of IHD in women might be due to the protective effects of natural estrogen and the hormonal shifts associated with menopause.42
There was no difference with respect to serum albumin levels, dialysis duration, frequency, and adequacy between the genders. This suggests that there was no difference between the genders in the quality of treatment delivered, which is similar to previous studies.43,44 These previous studies showed that there was no gender difference in self-care efficacy among HD population.
The current study is subject to a few limitations. Firstly, the findings of this study demonstrate a correlation between factors associated with mortality in dialysis patients, but they do not establish a cause-and-effect relationship. Secondly, it was a cross-sectional data collection. Hence, variables like BMI, comorbidity status, and lab results, may vary with time. Thirdly, the southern states of India consistently excel compared to the rest of the nation in terms of health, education, and economic prospects.45 Disproportionately higher representation of the South Indian population in this study could have confounded the observations. Fourthly, the sample size of women being much smaller than the men is bound to have confounded association of some factors with mortality as in men in this population. Lastly, the sex of the participants was recorded from medical records, without accounting for transgender or non-binary individuals.
Males predominated on maintenance hemodialysis. Females were less educated, required temporary HD catheters for initiation, and less likely to be paid for by public or private health insurance cover compared to males. Hypertension and IHD were significantly more prevalent in males, while diabetes as the cause of kidney failure was more prevalent in females. Multivariate analysis demonstrated that lower educational status, hypoalbuminemia, previous history of hospitalization (last 3 months), and dialysis in centers run by PPP were associated with mortality in males. Lower educational status, heart failure, and previous history of hospitalization were associated with mortality in females.
Acknowledgements
We thank NephroPlus to providing the data used in this study and to the patients treated at NephroPlus centres who consented for their data to be used for research purposes.
Conflicts of interest
There are no conflicts of interest.
References
- The rise and fall of women’s advantage: a comparison of national trends in life expectancy at age 65 years. Eur J Ageing. 2013;10:271-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiology of coronary heart disease in women. Heart. 2006;92:iii2-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Exploring sex disparities in cardiovascular disease risk factors using principal component analysis and latent class analysis techniques. BMC Med Inform Decis Mak. 2023;23:101.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sex differences in type 2 diabetes. Diabetologia. 2023;66:986-1002.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019;17:136.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Deaths: Leading Causes for 2017.2019. Accessed September 17, 2022. https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_06-508.pdfGoogle Scholarhttps://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)Google Scholar
- Sex differences in acute coronary syndromes: a global perspective. J Cardiovasc Dev Dis. 2022;9:239.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Major depressive disorder and difference between genders. Mater Sociomed. 2021;33:105-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sex differences in acute kidney injury requiring dialysis. BMC Nephrol. 2018;19:131.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- RENAL study investigators and the Australian and New Zealand intensive care clinical trials group. Sex and mortality in septic severe acute kidney injury. J Crit Care. 2019;49:70-6.
- [CrossRef] [PubMed] [Google Scholar]
- Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res. 2010;33:383-92.
- [CrossRef] [PubMed] [Google Scholar]
- Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14:151-64.
- [CrossRef] [PubMed] [Google Scholar]
- NECOSAD Study Group. Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant. 2011;26:270-6.
- [CrossRef] [PubMed] [Google Scholar]
- Gender and human chronic renal disease. Gend Med. 2008;5:S3-S10.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319-29.
- [CrossRef] [PubMed] [Google Scholar]
- The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis. 2005;46:863-70.
- [CrossRef] [PubMed] [Google Scholar]
- The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375-82.
- [CrossRef] [PubMed] [Google Scholar]
- Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS) PLoS Med. 2014;11:e1001750.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sex-specific analysis of haemodialysis prevalence, practices and mortality over time: the Austrian Dialysis Registry from 1965 to 2014. Nephrol Dial Transplant. 2019;34:1026-35.
- [CrossRef] [PubMed] [Google Scholar]
- Fundamentals of Biostatistics (5th ed). Duxbury; 2000. p. :80-240. [ISR1]
- Statistics in Medicine (2nd ed). Academic Press; 2005. p. :85-125.
- An introduction to biostatistics, A manual for students in health sciences (4th edition). New Delhi: Prentice Hall of India; 2006. p. :86-160.
- Sample size estimation and power analysis for clinical research studies. J Human Reprod Sci. 2012;5:7-13.
- [CrossRef] [Google Scholar]
- Mortality Rate, Adult, (per 1,000 Adults) - India. World Bank Open Data; 2021. https://data.worldbank.org/indicator/SP.DYN.AMRT.MA?locations=IN
- Sex disparities in mortality among patients with kidney failure receiving dialysis. Sci Rep. 2022;12:18555.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016.
- BMI and its association with death and the initiation of renal replacement therapy (RRT) in a cohort of patients with chronic kidney disease (CKD) BMC Nephrol. 2019;20:329.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical Research Center for End Stage Renal Disease (CRC for ESRD) Investigators. Survival in patients on hemodialysis: effect of gender according to body mass index and creatinine. PLoS One. 2018;13:e0196550.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gender-specific discrepancy in subjective global assessment for mortality in hemodialysis patients. Sci Rep. 2018;8:17846.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gender-specific associations of skeletal muscle mass and arterial stiffness among peritoneal dialysis patients. Sci Rep. 2018;8:1351.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- ATTOM investigators. Health literacy and patient outcomes in chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2018;33:1545-58.
- [CrossRef] [PubMed] [Google Scholar]
- Gender disparity in health and food expenditure in India among elderly. Int J Popul Res 2014
- [CrossRef] [Google Scholar]
- Gender differences in the use of health care in China: cross-sectional analysis. Int J Equity Health. 2014;13 10.1186/1475-9276-13-8
- [CrossRef] [Google Scholar]
- Gender differences in health care expenditures, resource utilization, and quality of care. J Manage Care Pharm. 2008;14:S2. Available: http://www.amcp.org/data/jmcp/JMCP Supp_April08_S2-S6.pdf. [PMC free article] [PubMed] [Google Scholar]
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Age and gender differences in medicare expenditures and service utilization at the end of life for lung cancer decedents. Women’s Health Issues. 2008;18:199-209.
- [CrossRef] [PubMed] [Google Scholar]
- Gender differences in utilization of preventive care services in the United States. J Women’s Health. 2012;21:140-5.
- [CrossRef] [PubMed] [Google Scholar]
- Periodic Labor Force Survey (PLFS) Annual Report 2022-2023 Released [Internet]. Available from: https://pib.gov.in/PressReleaseIframePage.aspx?PRID = 1966154
- Social roles and physical health: the case of female disadvantage in poor countries. Social Sci Med. 1995;40:147-61.
- [CrossRef] [PubMed] [Google Scholar]
- Gender difference in health-care expenditure: evidence from India human development survey. PLoS One. 2016;11:e0158332.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Are women more susceptible to ischemic heart disease compared to men? A literature overview. J Geriatr Cardiol. 2021;18:289-96.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The evaluation of self-care and self-efficacy in patients undergoing hemodialysis. J Eval Clin Pract. 2010;16:605-10.
- [CrossRef] [PubMed] [Google Scholar]
- Self-care on hemodialysis: behaviors with the arteriovenous fistula. Ther Apher Dial. 2017;21:195-9.
- [CrossRef] [PubMed] [Google Scholar]
- BBC News. (n.d.-b). Why south India outperforms the north. https://www.bbc.com/news/world-asia-india-62951951








