Translate this page into:
Glomerulonephritis After Renal Transplatation in South Asia - Single Center Experience Over 5 Decades
Corresponding author: Suceena Alexander, Department of Nephrology, Christian Medical College Vellore, Ranipet Campus, Ranipet, India. E-mail: suceena@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Yusuf S, Alexander S, Roy S, Rebekah G, John EE, Thomas A, et al. Glomerulonephritis After Renal Transplantation in South Asia - Single Center Experience Over 5 Decades. Indian J Nephrol. 2025;35:270-6. doi: 10.25259/IJN_39_2024
Abstract
Background
With significant advances in the understanding of transplant immunology and a reduction in rejection rates, significant improvements in kidney allograft survival have been seen. The problem of recurrent and denovo glomerular diseases after transplantation affecting graft outcomes remains and is poorly characterized. This study aimed to analyze the incidence, characteristics, and outcomes of glomerulonephritis (GN) after kidney transplant in the Indian subcontinent.
Materials and Methods
Data on patients who underwent kidney transplants in our hospital from 1971 to 2018 was analyzed. Patients who had biopsy proven glomerulonephritis after transplant were included in the study. Demographic factors, characteristics of glomerulonephritis after transplant, and patient and graft outcomes were studied.
Results
Post-transplant glomerulonephritis was seen in 177 out of 3630 (4.8%) patients. IgA nephropathy (IgAN) was the most common type, followed by focal segmental glomerulosclerosis (FSGS) and thrombotic microangiopathy (TMA). Patients with IgAN and FSGS were younger, and native kidney disease was unknown in the majority (70% in IgAN and 40% in FSGS). Glomerulonephritis was the most common cause of graft loss. A serum creatinine level of ≥2 mg/dL at 1 year post-transplant was significantly associated with the risk of death and graft loss. In addition, the occurrence of glomerulonephritis within a year of transplant and cytomegalovirus (CMV) infection were found to be significant risk factors for death and graft loss, respectively.
Conclusion
Post transplant glomerulonephritis can significantly impact patient and graft outcomes. Understanding its etiology and pathogenesis is crucial to enabling its prevention and management and improving the outcomes of kidney transplantation.
Keywords
Death
Glomerulonephritis
Graft survival
Kidney transplantation
Recurrence
Introduction
Glomerulonephritis (GN), one of the major causes of end-stage kidney disease (ESKD) worldwide,1 is an important cause of allograft loss associated with a significant reduction in graft survival. Considering the observed variation in the epidemiology and outcomes of glomerular diseases in the native kidneys in different parts of the world, differences may exist in post-transplant glomerular diseases as well. The first and largest study of post-transplant glomerulonephritis in the Indian subcontinent, we examine the incidence, types, and timeline of post-transplant glomerulonephritis and determine the factors associated with the risk of death and graft loss.
Materials and Methods
Data was collected from electronic (Clinical WorkStation) and paper hospital records of all the renal allograft recipients (RARs) who underwent kidney transplantation in our hospital from 1971 to 2018. All patients who had biopsy proven glomerulonephritis after kidney transplantation were included in the study. This included patients with either a recurrent glomerulonephritis, de novo glomerulonephritis or glomerulonephritis with unknown native kidney disease (NKD). Recurrent glomerulonephritis was defined as the presence of the same biopsy proven glomerular disease in the native kidneys and the graft. De novo glomerulonephritis was defined as the presence of biopsy proven kidney disease in the allograft different from the NKD. Glomerulonephritis with unknown NKD was defined as the presence of a biopsy proven glomerular disease in the allograft without histological confirmation of NKD. Data was collected from patients until their last follow-up or death. Institutional review board approval has been obtained. Patients who were lost in follow-up before the completion of one year post-transplant were excluded from the study. Appropriate patient consent was obtained.
Demography, incidence, spectrum, and timeline of glomerulonephritis after kidney transplantation were explored. The primary outcomes were patient and/or graft survival. Risk factors associated with death and graft loss were studied. Death censored graft loss was defined as the need for kidney replacement therapy in the form of dialysis or repeat kidney transplantation.
All statistical analyses were performed using IBM SPSS Statistics version 21. Descriptive statistics were reported using mean± SD for continuous variables, median (IQR) for skewed variables, and frequency and percentage for categorical variables. Chi-square/Fisher’s exact test was used to check the association. Kaplan Meier analysis was done to assess the survival curve, and they were compared with Log Rank (Mantel-Cox). Cox regression was done to assess the multivariate analysis after checking for Cox proportional hazard model.
Results
Between 1971 and 2018, 3630 patients underwent kidney transplantation of which 177 (4.8%) had biopsy proven glomerulonephritis after transplantation. The most common types of glomerulonephritis were IgAN (n = 93, 52%), followed by focal segmental glomerulosclerosis (FSGS) (n = 37, 21%) and thrombotic microangiopathy (TMA) (n = 28, 16%) [Figure 1]. The baseline characteristics and outcomes of the three most common types of glomerulonephritides are presented in Table 1.
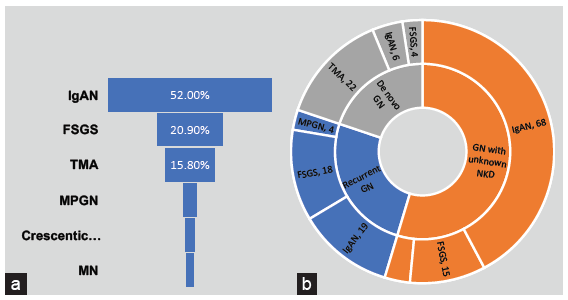
- Burden of disease (a) Frequency of different types of glomerulonephritis seen after renal transplant. (b) Distribution of categories and types of GN after transplant. MPGN: Membranoproliferative glomerulonephritis, MN: Membranous nephropathy, IgAN: IgA nephropathy, FSGS: Focal segmental glomerulosclerosis, TMA: Thrombotic microangiopathy, NKD: Native kidney disease, Recurrent GN: Recurrent glomerulonephritis, De novo GN: De novo glomerulonephritis.
| Baseline characteristics and outcomes | IgAN (n = 93) | FSGS (n = 37) | TMA (n = 28) |
|---|---|---|---|
| Categories of glomerulonephritis (GN) | |||
| GN with NKD unknown | 68 (73%) | 15 (40%) | 4 (14%) |
| Recurrent GN | 19 (20%) | 18 (49%) | 2 (7%) |
| De Novo GN | 6 (6%) | 4 (11%) | 22 (78%) |
| Mean age (SD) in years | 30 (9) | 29 (11) | 37 (12) |
| Gender | |||
| Male | 81 (87%) | 27 (73%) | 22 (79%) |
| Female | 12 (13%) | 10 (27%) | 6 (21%) |
| Pre transplant diabetes | 3 (3%) | 2 (5%) | 5 (18%) |
| Pre transplant TB | 14 (15%) | 5 (13%) | 1 (4%) |
| Pre transplant hepatitis | |||
| Hepatitis B | 2 (2%) | 3 (8%) | 0 |
| Hepatitis C | 5 (5%) | 1 (3%) | 1 (4%) |
| Median duration on dialysis (IQR) in months | 4 (5) | 3 (3) | 3 (3) |
| Donor sex | |||
| Male | 35 (38%) | 6 (17%) | 14 (50%) |
| Female | 56 (61%) | 30 (83%) | 13 (46%) |
| Mean donor age (SD) in years | 41 (11) | 41 (11) | 48 (9) |
| Donor relation | |||
| Spousal | 9 (10%) | 4 (11%) | 6 (21%) |
| First degree | 74 (80%) | 28 (76%) | 18 (64%) |
| Second degree | 7 (7%) | 3 (8%) | 3 (11%) |
| Deceased | 3 (3%) | 2 (5%) | 1 (4%) |
| Nature of induction | |||
| None | 39 (42%) | 21 (57%) | 11 (39%) |
| Basiliximab | 47 (50%) | 15 (40%) | 16 (57%) |
| Thymoglobulin | 7 (7%) | 1 (3%) | 1 (4%) |
| Maintenance immunosuppression | |||
| Cyclosporine | 34 (37%) | 21 (57%) | 15 (54%) |
| Tacrolimus | 53 (57%) | 16 (43%) | 10 (36%) |
| Azathioprine | 32 (34%) | 15 (40%) | 8 (29%) |
| MPA | 57 (61%) | 19 (51%) | 19 (68%) |
| CNI withdrawal | 22 (24%) | 7 (19%) | 17 (61%) |
| Use of CMV prophylaxis | 52 (56%) | 16 (43%) | 9 (32%) |
| Median time (SD) to detection of GN in months | 46 (49) | 16 (45) | 1.5 (12) |
| Indication for kidney bx | |||
| Proteinuria | 71 (77%) | 35 (100%) | 5 (18%) |
| Active urine sediment | 39 (42%) | 6 (17%) | 0 |
| Rise in creatinine | 39 (42%) | 23 (66%) | 28 (100%) |
| Post transplant infections | |||
| Hepatitis B | 4 (4%) | 2 (5%) | 1 (4%) |
| Hepatitis C | 2 (2%) | 1 (3%) | 1 (4%) |
| CMV | 15 (16%) | 4 (11%) | 6 (21%) |
| BKV | 7 (7%) | 2 (5%) | 1 (4%) |
| EBV | 1 (1%) | 1 (3%) | 0 |
| Tuberculosis | 10 (11%) | None | 6 (21%) |
| NODAT | 22 (24%) | 7 (19%) | 7 (25%) |
| BPAR | 29 (31%) | 20 (54%) | 8 (28%) |
| Post transplant malignancy | 2 (3%) | 1 (3%) | 1 (4%) |
| Median (IQR) creatinine at 1 year (mg/dl) | 1.3 (0.4) | 1.6 (0.8) | 1.4 (1.07) |
| Death | 2 (2%) | 5 (13%) | 9 (32%) |
| Graft loss | 14 (15%) | 17 (46%) | 10 (36%) |
| Causes of graft loss | |||
| Non compliance | 2 (14%) | 1 (6%) | 0 |
| Chronic rejection | 6 (43%) | 2 (12%) | 3 (30%) |
| Chronic pyelonephritis | 1 (7%) | 0 | 0 |
| Glomerulonephritis | 4 (28%) | 14 (82%) | 7 (70% |
| BKVN | 1 (7%) | 0 | 0 |
CMV: Cytomegalovirus, CNI: Calcineurin inhibitor, FSGS: Focal segmental glomerulosclerosis, IgAN: IgA nephropathy, MPA: Mycophenolic acid, NKD: Native kidney disease, NODAT: New onset diabetes after transplant, TB: Tuberculosis, TMA: Thrombotic microangiopathy, bx: biopsy, BKV: BK virus, BKVN: BK virus nephropathy, BPAR: Biopsy proven acute rejection, EBV: Epstein Barr virus, IQR: Interquartile range, SD: Standard deviation.
IgAN: IgAN was the most common type of glomerulonephritis after transplantation accounting for half the number of cases. In IgAN, the most common category was IgAN with unknown NKD (n = 68, 73%), followed by recurrent IgAN (n = 19, 21%) and de novo IgAN (n = 6, 6%). In the six patients with de novo IgAN, the NKD was glomerulonephritis in three (FSGS, mesangioproliferative and immune complex mediated glomerulonephritis) and diabetic nephropathy in another three patients. Overall, the mean age of patients was 30±9 years. Patients with de novo IgAN were older (mean age 44 ± 12 years, p = 0.001) compared to those with IgAN with unknown NKD (mean age 29 ± 8 years) and recurrent IgAN (mean age 26 ± 6 years). The median time to detection of IgAN after kidney transplant was 46 (25,70) months, varying between 46 (24,67) months in IgAN with unknown NKD, 42 (25,68) months in recurrent IgAN, and 68 (57,170) months in de novo IgAN (p = 0.082). The most common presentation was the occurrence of proteinuria in more than 80% of patients. Active urinary sediment and graft dysfunction were seen in 40% patients. No significant difference in clinical presentation was noted between the different categories. Median (IQR) serum creatinine at 1 year was 1.3 (0.4) mg/dL and was found to be comparable in the three categories (p = 0.951). Graft and patient survival were also comparable. Overall graft loss was seen in 14 patients (15.1%). Graft loss was attributed to IgAN in 4 patients (28%).
FSGS: FSGS was seen in 37 (21%) patients; the most common category was recurrent FSGS (n = 18, 49%) followed by FSGS with unknown NKD (n = 15, 40%) and de novo FSGS (n = 4, 11%). Only patients with primary FSGS were included. Cases were classified as primary based on clinical presentation with nephrotic syndrome. An EM diagnosis of podocytopathy was available in seven patients.
In the four patients with de novo FSGS, the NKD was diabetic nephropathy, kidney calculus disease, and IgAN. The mean age of patients was 29 ± 11 years and was comparable across the three categories of FSGS (p = 0.5). Overall, the median time to detection of FSGS after transplant was 16 (3,28) months. Occurrence was earliest in recurrent FSGS 15 (1.7,31) months followed by FSGS with unknown NKD 16 (2,6) months but it was much later at 59 (6,41) months in de novo FSGS (p = 0.5). All the patients presented with proteinuria (nephrotic syndrome in 100%). Graft dysfunction was seen in 66% and active urinary sediment was seen only in 17% patients. The clinical presentation was not found to vary significantly between the various categories. Donors were first-degree relatives in majority (80%) of patients. Median (IQR) serum creatinine at 1 year was 1.5 (0.8) mg/dL with no significant variation across groups. The occurrence of graft loss and death did not show any significant difference between the three categories. Graft loss was seen in nearly 50% of patients, with FSGS in graft being the predominant cause of graft loss (14/17 (82%). Among the five patients who died, four (80%) died with functioning graft.
TMA: TMA was seen in 28 (16%) patients. De novo TMA was the predominant category (n = 22, 80%). The mean age of the patients was 37 ± 12 years. Maintenance immunosuppression consisted of cyclosporine in the majority 54% (vs tacrolimus in 36%), antiproliferative agent mycophenolic acid (MPA) in 68% (vs azathioprine in 29%). The occurrence of TMA was mostly within the first 2 weeks after transplantation (numbers and %). All the patients presented with graft dysfunction (100%); proteinuria was seen in a minority (15%) of patients. Most were attributed to calcineurin inhibitors (CNIs) and CNI was withdrawn in 61% patients and proliferation signal inhibitor (PSI) added in 54%. Graft loss was seen to occur in 10/28 (35.7%) and 7/10 cases (70%) were attributed to TMA.
Crescentic Glomerulonephritis: All five cases of crescentic glomerulonephritis received allografts before 1996. NKD was unknown in all of them. None of the patients received induction therapy and the maintenance IS consisted of cyclosporine and azathioprine. Two patients had immune complex mediated crescentic glomerulonephritis, one with crescentic IgA, one with anti-glomerular basement membrane disease while one was uncharacterized by no immunofluorescence. Median serum creatinine at 1 year after transplantation was 2.0 mg/dL.
Patient and Graft Survival: Overall graft loss occurred in 49 patients (n = 177, 28%). Glomerulonephritis was the most common cause of graft loss, accounting for nearly 60% cases (varying from 30% in IgAN to 80% in FSGS) followed by chronic rejection. Twelve percent of the patients died. The most common cause of death was sepsis (45%) followed by ESKD (18%) related complications. Cox proportional hazards analysis was done to determine the risk factors for death and graft loss. On univariate analysis, serum creatinine at 1 year, time of detection of glomerulonephritis after kidney transplant, cytomegalovirus (CMV) infection, and lack of use of CMV prophylaxis were found to be significant risk factors for death. On multivariable Cox proportional hazards analysis, serum creatinine level ≥ 2 mg/dL at one year post transplant and the occurrence of glomerulonephritis less than one year post transplant were significant risk factors for death, with hazard ratios of 15.3 (1.5–149.3) and 8.4 (1.1–62.1), respectively. For death-censored graft loss, significant risk factors on univariate analysis were time to detection of glomerulonephritis after kidney transplant, serum creatinine at 1 year, CMV infection, and absence of new onset diabetes after transplant (NODAT). After multivariable Cox proportional hazards analysis, serum creatinine of ≥ 2 mg/dL at one year post transplant (HR 4.8, 95% CI 1.6–14.2) and CMV infection (HR 2.8, 95% CI 1.1–7.1) remained risk factors. Patient and graft survival were also compared between IgAN and FSGS: the two most common types of glomerulonephritis detected in our cohort. Both were found to be significantly poorer in FSGS compared to IgAN [Figure 2].
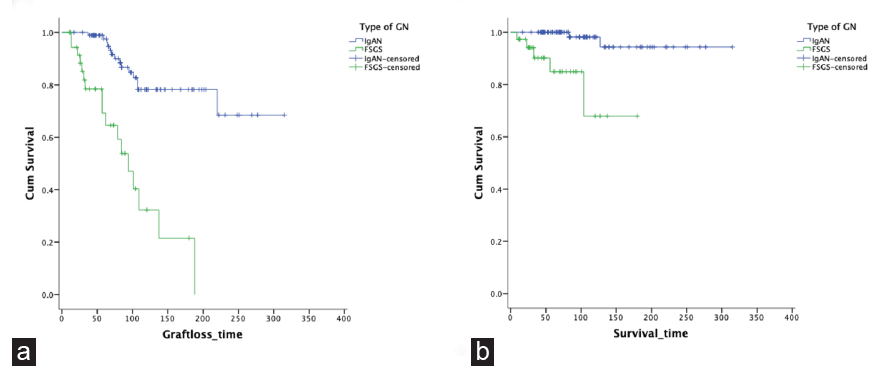
- Comparison of outcomes in IgAN vs FSGS after renal transplant. (a) Patient survival (b) Graft survival. GN: Glomerulonephritis, IgAN: IgA nephropathy, FSGS: Focal segmental glomerulosclerosis.
Discussion
Glomerulonephritis was seen to develop in nearly 5% of patients who underwent kidney transplantation at our center. Prevalence rates vary between 2% and 18%2-6 as reported in the literature. While most of the studies conducted on post transplantation glomerulonephritis have focused on recurrent glomerulonephritis only, our study included all three categories—recurrent glomerulonephritis, glomerulonephritis with unknown NKD, and de novo glomerulonephritis, since the majority (56%) of our patients with glomerulonephritis after transplantation were those in whom NKD was unknown. This, in our setup, reflects the advanced stage at which most of our patients seek medical attention. Similar to other studies,4,6,7 IgAN and FSGS were the two most common types of glomerulonephritis after transplantation.
Our patients who developed IgAN after transplantation were younger (mean age 30± 9 years) compared to that reported in other studies,8-10 highlighting the existent racial differences in the epidemiology and prognosis of this disease.11 The association of IgAN with living related donors has been addressed previously.12-14 Since donors were related to nearly 90% of our patients, the likelihood of genetic factors facilitating the development of IgAN in allografts needs to be studied further. The median time to detection of IgAN was 4 years after kidney transplantation, which is the same as described in studies from other parts of the world, with only a few studies demonstrating a median time as 6–7 years.10,12 IgAN as a cause of graft loss was seen in 28% of patients. Rates of graft loss caused by IgAN have been variably reported between 10% and 50% in different studies.15,16 As highlighted by Floege et al.,17 this variation may be attributed to the more rapid manifestation of chronic allograft nephropathy in some studies.
There is a paucity of data on de novo IgAN, with few studies and case series available to make comparisons. In our study, patients with de novo IgAN were significantly older and developed IgAN much later, with three out of six patients having pre-transplant diabetes and diabetic nephropathy. The occurrence of IgAN in patients with diabetes has always been of pathogenetic interest, and various mechanisms like increased immune complex deposition in the presence of hyperfiltration, intraglomerular hypertension, alteration of charge by glycated proteins, abnormally increased serum IgA level, all facilitating the localization of immunoreactants, have been proposed.18,19 Whether these mechanisms are aggravated in the graft kidney needs to be looked into. None of the patients with de novo IgAN suffered graft loss. The lack of pre-existing literature and the small number of cases limited our ability to arrive at specific conclusions.
Patients with FSGS after transplantation were also found to be younger (mean age 29 ± 11 years) compared to that reported in most other studies.20-24 Other studies in India on native kidneys25,26 have also reported younger patients compared to other populations. This leads us to postulate whether lower nephron endowment in South Asians predisposes to its earlier development in the graft kidney as well. Donors were first-degree relatives of three-fourths of our patients. The sharing of HLA antigens may influence the susceptibility to the development of FSGS after transplantation. The median time to detection of FSGS after kidney transplant was 16 months, which is later compared to that reported by others.20,22 Similar rates of graft loss (80% in our cohort) have been reported in other studies with recurrent FSGS after transplant.20,21,24,27
Most of the data on post-transplant TMA worldwide is limited to case reports and case series. In one of the largest analyses of USRDS by Reynolds et al.28 between 1998 and 2000, TMA was identified in 149 patients; majority having de novo TMA. Similarly, in our study, majority (80%) had de novo TMA. The mean recipient and donor ages in our study were comparable to those of Reynolds et al. and younger recipient and older donor were significantly associated with the risk of developing TMA.28,29 Although, a predominance of female patients has been seen in other studies, the same was not reflected in our study, probably because of the gender disparity in kidney transplants in this region. TMA occurred earlier after the transplant in our patients. CMV infection was seen in 21% of patients and tuberculosis (TB) developed in 21%. CMV infection has been associated with a risk of TMA, but there are no previous reports of TB associated with the development of TMA in the graft, though a few case reports of TMA in native kidneys and TB have been published. Increased procoagulant activity of IL-1 on endothelial cells is the proposed mechanism.30 However, TB developed at varied periods post-transplant while TMA occurred in the early post-transplant period; whether the presence of latent mycobacterial infection has any role in predisposing to TMA needs to be explored further. All patients with recurrent TMA and TMA associated with chronic rejection suffered graft loss, portending a poor prognosis in these patients compared to drug-induced TMA. We observed rates of graft loss similar to other studies of TMA.29
There is limited published literature on crescentic glomerulonephritis after kidney transplantation probably related to the rarity of its occurrence. We did not encounter any patients after 1996, which attributed to the increased use of MPA in the later decades after this.
Compared to our cohort (28%), higher rates of graft loss (45%–70%) have been reported in other studies.4,6 This difference might be explained by the disproportionately higher number of patients with IgAN in our cohort. The principal predictors of graft loss were found to be CMV infection and serum creatinine ≥ 2 mg/dL at 1 year post transplant. CMV has been described as induced glomerular injury, causing CMV glomerulopathy. Whether that may be intensified in the presence of underlying glomerular disease or whether the propensity of CMV to cause rejections or interstitial damage increases the risk of graft loss needs to be identified further. We identified serum creatinine ≥ 2mg/dL at 1 year post transplant and the occurrence of glomerulonephritis at less than 1 year after transplant as significantly associated with the risk of death.
Baseline characteristics and outcomes were found to be comparable in patients with recurrent glomerulonephritis and glomerulonephritis with unknown NKD, which might point towards the fact that patients with unknown NKD might actually be those with recurrent glomerulonephritis in whom a native kidney disease diagnosis was not established prior to transplant. On the other hand, we did find significant differences in baseline characteristics in patients with de novo glomerular diseases, highlighting its nature as a separate entity with probably different causative and pathogenetic factors that need to be elucidated; outcomes, however, were not found to vary.
The strengths are it is the largest study of glomerulonephritis after transplant to be reported from India, analyzing data spanning over half a century. More than 3000 patients were studied, and majority (60%) had a follow-up of more than 5 years, which is essential to study the development of glomerulonephritis after transplant and assess long-term outcomes.
The limitations of the study are its retrospective nature, the lack of knowledge of native kidney disease in a large number of patients and absence of electron microscopy findings for all patients. Also, the detection of only a small number of patients with de novo glomerulonephritis precluded us from drawing any significant conclusions from them.
The prevalence of glomerulonephritis after transplantation in our population is comparable to studies from other parts of the world, however unlike data from the rest of the world, the majority of our patients have an unknown native kidney disease. Our patients with IgAN and FSGS after transplantation are younger; the post-transplant course and outcomes are, however, not different. Glomerulonephritis is the predominant cause of allograft loss in these patients, and its earlier occurrence after transplant is a significant risk factor for death.
Conflicts of interest
There are no conflicts of interest.
References
- Recurrent glomerulonephritis after renal transplantation: An unsolved problem. Clin J Am Soc Nephrol. 2008;3:800-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recurrent glomerulonephritis in renal transplants: fourteen years’ experience. Nephrol Dial Transplant. 1989;4:730-4.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent and de novo renal disease after kidney transplantation with or without cyclosporine A. Am J Kidney Dis Off J Natl Kidney Found. 1991;17:524-531.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int. 2017;92:461-9.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent and de novo renal diseases after renal transplantation: a report from the renal allograft disease registry. Am J Kidney Dis Off J Natl Kidney Found. 1998;31:928-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Probability, predictors, and prognosis of posttransplantation glomerulonephritis. J Am Soc Nephrol JASN. 2009;20:843-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recurrent glomerulonephritis following renal transplantation and impact on graft survival. BMC Nephrol. 2018;19:344.
- [CrossRef] [PubMed] [Google Scholar]
- Graft failure of IgA nephropathy in renal allografts following living donor transplantation: predictive factor analysis of 102 biopsies. BMC Nephrol. 2019;20:446.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Outcome of 313 czech patients with IgA nephropathy after renal transplantation. Front Immunol. 2021;12:726215.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- IgA nephropathy after renal transplant: Recurrences and de novo cases. Transplant Proc. 2020;52:515-8.
- [CrossRef] [PubMed] [Google Scholar]
- Three-year clinical outcomes of the first South Asian prospective longitudinal observational IgA nephropathy cohort. Kidney Int Rep. 2022;7:305-18.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recurrent IgA nephropathy after kidney transplantation. Transplant Proc. 2016;48:2689-94.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence of IgA nephropathy among renal allograft recipients from living donors is greater among those with zero HLA mismatches. Transplantation. 2006;82:759-62.
- [CrossRef] [PubMed] [Google Scholar]
- Increased glomerulonephritis recurrence after living related donation. BMC Nephrol. 2017;18:25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Kidney transplantation in patients with IgA mesangial glomerulonephritis11See Editorial by Hogg, p. 2033. Kidney Int. 2001;60:1948-54.
- [Google Scholar]
- Recurrent IgA nephropathy after renal transplantation. Semin Nephrol. 2004;24:287-91.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent IgA nephropathy in the renal allograft: not a benign condition. Nephrol Dial Transplant. 2013;28:1070-3.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 1997;12:2588-91.
- [CrossRef] [PubMed] [Google Scholar]
- Elevation of serum IgA1 levels in patients with diabetic nephropathy. Nephron. 1993;63:355.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence of FSGS after kidney transplantation in adults. Clin J Am Soc Nephrol. 2020;15:247-56.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Long-term outcome of renal transplantation in adults with focal segmental glomerulosclerosis. Transpl Int. 2010;23:208-16.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab for recurrence of primary focal segmental glomerulosclerosis after kidney transplantation: Clinical outcomes. Transplantation. 2017;101:649-56.
- [CrossRef] [PubMed] [Google Scholar]
- Response to plasma exchange and graft survival in recurrent focal and segmental glomerulosclerosis after transplantation: Does the time of recurrence matter? A retrospective study. Transpl Int. 2021;34:302-12.
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective study of focal segmental glomerulosclerosis: Clinical criteria can identify patients at high risk for recurrent disease after first renal transplantation. BMC Nephrol. 2013;14:47.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Primary focal segmental glomerulosclerosis in adults: Is the Indian cohort different? Nephrol Dial Transplant. 2009;24:3701-7.
- [CrossRef] [PubMed] [Google Scholar]
- Primary FSGS in nephrotic adults: Clinical profile, response to immunosuppression and outcome. Nephron. 2016;132:81-5.
- [CrossRef] [PubMed] [Google Scholar]
- Long-Term outcome of kidney transplantation in recipients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2016;11:2041-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Thrombotic microangiopathy after renal transplantation in the United States1 1The opinions expressed are solely those of the authors and do not represent an endorsement by the Department of Defense or the National Institutes of Health.This is a US government work. There are no restrictions on its use. Am J Kidney Dis. 2003;42:1058-68.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and pathological features of thrombotic microangiopathy influencing long-term kidney transplant outcomes. PLOS ONE. 2020;15:e0227445.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Thrombotic thrombocytopenic purpura associated with primary tuberculosis. Infection. 1995;23:58-9.
- [CrossRef] [PubMed] [Google Scholar]








